Across all of medicine, the study of individuals with an early onset of a multifactorial disorder has been an important approach used to minimize heterogeneity and possibly identify unique or more salient risk factors. Since 1990, a study of childhood-onset schizophrenia (onset of psychotic symptoms by age 12) has been ongoing at the National Institute of Mental Health (NIMH)
(1). These clinical and neurobiological studies have provided support for the continuity of childhood-onset schizophrenia with later-onset schizophrenia
(2). The fact that childhood-onset schizophrenia is often chronic and refractory and that it may be associated with higher rates of familial “schizophrenia spectrum disorders” has led to the speculation that this form of the disorder may represent a particularly severe variant with a stronger genetic predisposition
(2).
Perhaps because of its relative rarity, childhood-onset schizophrenia is frequently misdiagnosed
(3). As part of our search for subjects with childhood-onset schizophrenia, it became apparent from chart review or in-person examinations that most patients with a referring diagnosis of childhood-onset schizophrenia had other disorders
(3). Within this group, a relatively large number of children with severe emotional disturbances reported brief, intermittent psychotic symptoms that did not meet the DSM-III-R/DSM-IV duration or severity criteria for schizophrenia.
Children with atypical or subclinical psychotic symptoms have been well recognized but difficult to classify
(4,
5). Because of the clinical heterogeneity of this group, a subgroup from the larger population of children with psychotic disorder not otherwise specified, provisionally labeled “multidimensionally impaired,” was studied as a parallel group to the childhood-onset schizophrenia group
(3,
6,
7). Children from this psychotic disorder not otherwise specified subgroup shared a number of phenomenological similarities with the childhood-onset schizophrenia patients, including prepsychotic linguistic deficits
(6), higher rate of schizophrenic spectrum disorders in first-degree relatives
(6), a generalized pattern of cognitive deficits
(8), and quantitative structural brain abnormalities (e.g., enlarged lateral ventricles and smaller total cerebral volume) seen at initial magnetic resonance imaging (MRI) scan
(9). In contrast, a 2–8 year prospective clinical follow-up study of 27 of these subjects with this psychotic disorder not otherwise specified subtype showed that psychotic symptoms had been transient and that none had progressed to schizophrenia
(7). Moreover, at follow-up about 50% of these subjects met criteria for an affective disorder
(7).
Eye-tracking dysfunction characterized by a disturbance in the smooth pursuit system has been found in 21%–85% of schizophrenic patients and in 8% of the normal population
(10,
11). Similar eye-tracking dysfunction has also been observed in 19%–34% of the biological first-degree relatives of schizophrenic patients
(11–
14) and in children of schizophrenic patients
(15). Studies of psychotic patients have yielded inconsistent results about whether smooth pursuit eye-tracking dysfunction is specific to schizophrenia
(11,
12,
14) or if it is associated with other nonaffective psychoses
(11,
16–
18). These discrepant findings may reflect problems with sample size or varying diagnostic criteria or methodology across studies
(11).
In a preliminary report, the oculomotor performance of our first 17 patients with childhood-onset schizophrenia (onset of psychotic symptoms before age 12) was characterized by significantly lower gain (ratio of eye velocity to target velocity), higher root mean square error (extent to which eyes do not reproduce target motion), lower percentage of total task time engaged in tracking the target, and a greater frequency of anticipatory saccades than healthy comparison subjects
(19). In contrast, there were no significant differences in gain scores or any saccade frequency between subjects with attention deficit hyperactivity disorder (ADHD) and healthy comparison subjects, although the ADHD subjects had higher root mean square error. This study was limited, however, by the use of two different recording systems and the lack of a qualitative rating system to characterize smooth pursuit eye tracking. More recently, similar abnormalities were found in a separate group of 10 childhood-onset schizophrenia subjects for whom higher rates of familial eye-tracking abnormalities were also seen
(20).
In this report we focus on one specific neurobiological trait—eye-tracking dysfunction—as part of a program examining the neurobiological characteristics associated with childhood-onset schizophrenia and psychotic disorder not otherwise specified to help differentiate them from each other. The present study compared the oculomotor performance of an expanded cohort of 29 subjects with childhood-onset schizophrenia to that of 20 healthy comparison subjects similar in age distribution and gender proportion. In addition, the smooth pursuit eye tracking of a subgroup of 26 subjects with psychotic disorder not otherwise specified was compared to a separate group of 18 healthy subjects also matched for age and gender to see if the between-group differences resembled those seen with childhood-onset schizophrenia.
Method
Subjects
The recruitment and diagnostic procedures have been described previously
(3). These are summarized briefly herein, and the methods for the eye-tracking procedure are given in more detail. All subjects and their parents gave informed assent/consent before participation. This protocol was approved by the NIMH Internal Review Board.
Male and female patients age 6 to 18 diagnosed with schizophrenia whose onset of psychotic symptoms occurred by age 12 were recruited through national professional and patient advocacy organizations for participation in an inpatient trial of an atypical neuroleptic. A premorbid IQ of less than 70 and the presence of active medical or neurological disorders were exclusionary. A primary diagnosis of pervasive developmental disorder, dissociative disorder, mood disorder, conduct disorder, or personality disorder was also exclusionary.
From 950 referrals, over 600 charts were reviewed, and 200 patients and their families were seen in person. Admission to the study followed a detailed screening examination, which included a review of old charts and clinical and structural interviews with parents and child.
Childhood-Onset Schizophrenia Subjects
Seventy children and adolescents were diagnosed at the screening examination with childhood-onset schizophrenia, but six patients did not participate because of clinical reasons (e.g., loss of residential placement, parental reluctance). Of the 64 remaining subjects, 34 underwent the eye-tracking task, but three patients were subsequently excluded because of poor tracings resulting from either machine failure or excessive head motion. The records for two additional patients were excluded from this analysis because of inattention or lack of motivation during the task determined by visual inspection of the tracing. Thus, data for 29 subjects with childhood-onset schizophrenia are included in this report. To optimize performance, subjects were tested during the last week of an atypical neuroleptic trial. Of these 29 patients, 15 were receiving medications known to affect eye-tracking performance such as clozapine or a benzodiazepine
(10,
21,
22). The remaining patients were being treated with olanzapine or a typical neuroleptic.
Psychotic Disorder Not Otherwise Specified Subjects
Thirty-one children who were referred with a diagnosis of childhood-onset schizophrenia for an inpatient trial of an atypical neuroleptic were felt to fall under DSM-III-R or DSM-IV criteria for psychotic disorder not otherwise specified and were labeled by our group as being “multidimensionally impaired”
(3). Based on 71 consecutive referrals to the NIMH childhood-onset schizophrenia study, this multidimensionally impaired subgroup was reliably diagnosed (kappa=0.81) according to empirically derived criteria
(3) and was distinguishable from children with overlapping clinical presentations (e.g., ADHD, bipolar disorder, nonautistic forms of pervasive developmental disorder). This psychotic disorder not otherwise specified subgroup reported brief hallucinations and delusions that occurred a few times a month, typically under stress, and showed no evidence of thought disorder
(3). Although their psychotic symptoms were ego-dystonic and impaired their functioning, these intermittent problems were less the focus of concern than were their dramatic mood outbursts and periodic aggression, which had necessitated frequent psychiatric hospitalizations. These children lacked the striking and pervasive disturbances in cognition, defective emotional rapport, bizarre hallucinations, and delusions that characterized the subjects with childhood-onset schizophrenia. They were socially deficient but eagerly sought social interactions and were distressed by their frequent peer rejection. Although the distorted reality and affective lability of this group suggested borderline or schizotypal personality disorder, none of the children given a diagnosis of psychotic disorder not otherwise specified met criteria for these disorders
(3,
6).
Five children with psychotic disorder not otherwise specified did not participate in this study because of clinical considerations (previous head trauma [N=1], age below 8 at time of testing [N=1]) or poor tracings resulting from machine failure or excessive head motion (N=3).
Smooth pursuit eye-tracking data were obtained for 26 subjects with psychotic disorder not otherwise specified (22 male, four female). The testing for the patients was completed during the first 2 weeks of their inpatient admission while they were on a stable medication regimen. Of the psychotic disorder not otherwise specified patients, two were receiving medications (lithium or carbamazepine) known to affect eye-tracking performance
(10) as part of their medication regimen.
Healthy Volunteers
Healthy comparison subjects for both patient groups were selected from an ongoing NIMH study of brain development in children and adolescents
(23). Any physical or neurological disorder, a lifetime history of any psychiatric disorder, or a history of a major psychiatric disorder in first-degree relatives was exclusionary. For this carefully screened group approximately one in six contacts were accepted for the study. From a pool of 38 healthy volunteers who agreed to participate in this study, 18 subjects were selected as comparison subjects for the psychotic disorder not otherwise specified group, and 20 subjects were selected as comparison subjects for the childhood-onset schizophrenia group on the basis of age and gender. All volunteers were seen as outpatients.
Behavioral, Cognitive, and MRI Assessments
From the literature, the following clinical and neurobiological measures were available for correlative analyses with eye-tracking variables: age at study
(24), full-scale IQ
(25), and clinical ratings of symptom severity from the Scale for the Assessment of Negative Symptoms
(26,
27). Exploratory analyses of neuropsychological data (tests that measured six cognitive domains: speeded visual-motor processing and attention, abstraction-flexibility, verbal intelligence and language, spatial organization, verbal learning, and visual learning)
(8) and eye-tracking measures were also planned for this study.
Eye-Tracking Procedure
Eye movements were recorded in a quiet, darkened room with an infrared limbus detection eye-tracking device (Eye Trac Model 210, Applied Science Laboratories, Waltham, Mass.). Subjects wore goggles with infrared sources and sensors mounted on the inside. The sensors require no mechanical calibration, making the device convenient for use with subjects. This device has a response time constant of 4 msec, an accuracy of better than 0.25 of visual angle, and a range of 15° to the left and right of center. Electronic calibration was accomplished by having the subject fixate on a series of sequentially presented white square targets (each subtending a visual angle of less than 2.5°) displayed on a black background for 2 seconds at 7.5° intervals horizontally across the video monitor. This calibration procedure was run at the beginning of each eye movement task. The subjects were seated 57 cm away from a computer monitor on which a small bright white target was displayed against a black background. The subjects’ heads were secured by a biteplate. Analog output of the infrared tracking device was sampled at 1000 Hz by using an 8-bit analog-to-digital converter and was stored for subsequent analysis.
Before each trial, the subjects were given a verbal description of the task followed by a demonstration on the computer monitor while the procedure was described again. During this description, subjects were instructed to keep their eyes on the target and follow it as closely as possible.
Eye tracking was recorded during a smooth pursuit task in which subjects followed a target moving horizontally across the screen at a constant velocity of 17°/second. The target stopped 15° to the left and right of center and remained stationary for approximately 1 second at the end of each ramp. The constant horizontal velocity combined with fixation points at the end of each ramp created a trapezoidal waveform of target and eye motions. The fixation points between each ramp were used for further calibration before data analysis.
Each trial consisted of 10 full ramps with a partial ramp before the first full ramp and following the last full ramp. The two half-ramps were excluded from the analysis, leaving 10 ramps for quantitative and qualitative analysis.
Eye Movement Analysis
Eye movement data were analyzed with pattern-recognition computer software described elsewhere
(28,
29). The raw data consisted of eye- and target-position values for each millisecond of recorded tracking, which could be displayed on a computer screen as a plot of eye position versus time. Since the initiation of smooth pursuit is quite different from its maintenance, we excluded the 250 msec preceding or following changes in target direction from the analysis. Sequential manipulations were performed on the remaining data to isolate saccades from true pursuit and artifacts. First, for each millisecond the velocity of the tracking was calculated by subtracting the position value at 5 msec before the given point from the position value at 5 msec after the given point and dividing the result by 10 msec. The next step was identifying the saccades. This was done by using an automated procedure that labeled as saccades local peaks in eye velocity that were at least twice the variance of the local velocity. This approach allows reliable identification of saccades as small as 0.4° even during smooth pursuit. Presaccadic and postsaccadic position errors were determined 5 msec before the beginning and 5 msec after the end of the saccade, respectively. High-velocity intervals with a peak velocity greater than 850°/second or those that were immediately followed by another high-velocity interval in the opposite direction were discarded as artifacts. Both phases of any biphasic pattern were discarded, since preliminary studies (in which subjects purposely blinked) indicated that this pattern represents eye blinks. Following this computer analysis, each file was checked by the raters for missed blinks or artifacts or inappropriately labeled artifacts. Those areas determined to be head movement or moments of inattention were manually labeled as artifacts before the final computer calculations were completed. All intervals of eye tracking not meeting the above criteria for being high-velocity interval or artifact were considered to be smooth pursuit eye movement. Since we were interested in measuring smooth pursuit eye movement only when actively pursued targets were properly visualized by the fovea, we excluded from analysis intervals in which smooth pursuit eye movement velocity was less than 5°/second or smooth pursuit eye movement position error (eye position subtracted from target position) was greater than 2.5°, which is the radius of the fovea in humans.
Quantitative eye movement variables and saccade classification system
Quantitative variables were defined as follows. Smooth pursuit eye movement gain was defined as the ratio of eye velocity to target velocity of each separate smooth pursuit eye movement interval, averaged across intervals. Saccades were obtained by tabulating the number of all intervals considered to be saccades according to the criteria stated above (i.e., high-velocity intervals with simple uniphasic pattern). In addition, saccades were subclassified as catch-up and anticipatory saccades by their direction in relation to target motion and presaccadic and postsaccadic position errors. Saccades in the direction of the target motion that started and ended behind the target, and saccades that ended ahead of the target but with at least two-thirds of their saccadic amplitude-moving gaze toward the target, were classified as catch-up saccades. Saccades in the direction of target motion that ended ahead of the target and were followed by a 50 msec interval of eye velocity of less than 5°/second were classified as anticipatory saccades. Anticipatory saccades that were greater than 4° were subclassified as large anticipatory saccades. Saccade rates were determined by dividing the number of saccades by the pursuit duration.
Root mean square error, which provides a global measure of the extent to which the eyes do not reproduce target motion
(30), was calculated by squaring the difference between eye position and target position for all intervals of nonartifactual pursuit tracking, and then obtaining the average of the square root of these numbers. Split-half reliability (intraclass correlation coefficient [ICC]) for quantitative variables was high (ICC=0.75–0.96).
Qualitative eye movement analysis
Each ramp was scored qualitatively by one of the authors (R.N.) blind to patient identity on a scale of 1 (best) to 5 (worst)
(31). Two of the authors (R.N., A.S.) rated a subset of this sample (N=10) with high reliability (ICC=0.98). The average of 10 ramps was determined for each subject and used as the primary qualitative measure of eye-tracking dysfunction. Additionally, subjects with a mean score of 2.5 or greater were classified as having abnormal eye tracking. Qualitative ratings for all 93 subjects (26 with psychotic disorder not otherwise specified, 29 with childhood-onset schizophrenia, 38 healthy volunteers) were highly correlated with gain (r=0.83, df=91, p<0.001) and with root mean square error (r=0.77, df=91, p=0.0001).
Data Analyses
Analyses were performed with SPSS statistical analysis software
(32). To examine demographic differences between psychotic disorder not otherwise specified patients and healthy comparison subjects, t tests or chi-square analyses were used depending on data distribution and type. Distributions for all eye-tracking variables were examined within each group for significant skew, kurtosis, and heterogeneity of variance. Because the data for three of six eye-tracking variables from both the psychotic disorder not otherwise specified patients and the healthy volunteers were not normally distributed, data were analyzed by using the Mann-Whitney U test.
Spearman rank correlation coefficients, with partial correction for age, tested relationships between eye-tracking measures that differed significantly from those of healthy volunteers and clinical measures that might be related to eye-tracking performance.
Results
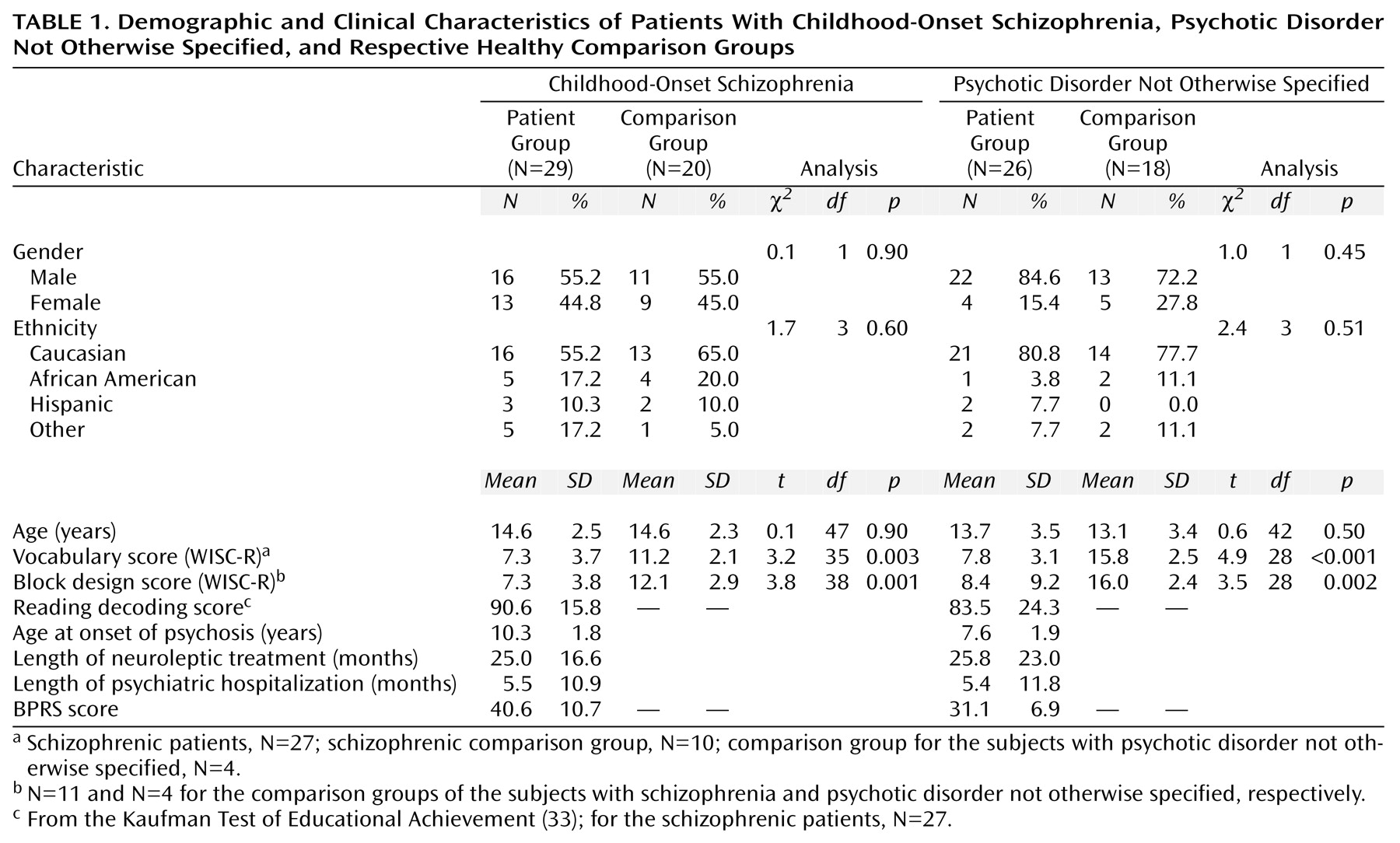
As seen in
Table 1, the patients with childhood-onset schizophrenia and psychotic disorder not otherwise specified performed more poorly on the WISC-R vocabulary and block design subtests than did the healthy volunteers. Because there were no significant group differences in age or gender distribution between the two patient groups and their respective healthy volunteers, comparative analyses are unadjusted.
Eye-Tracking Performance
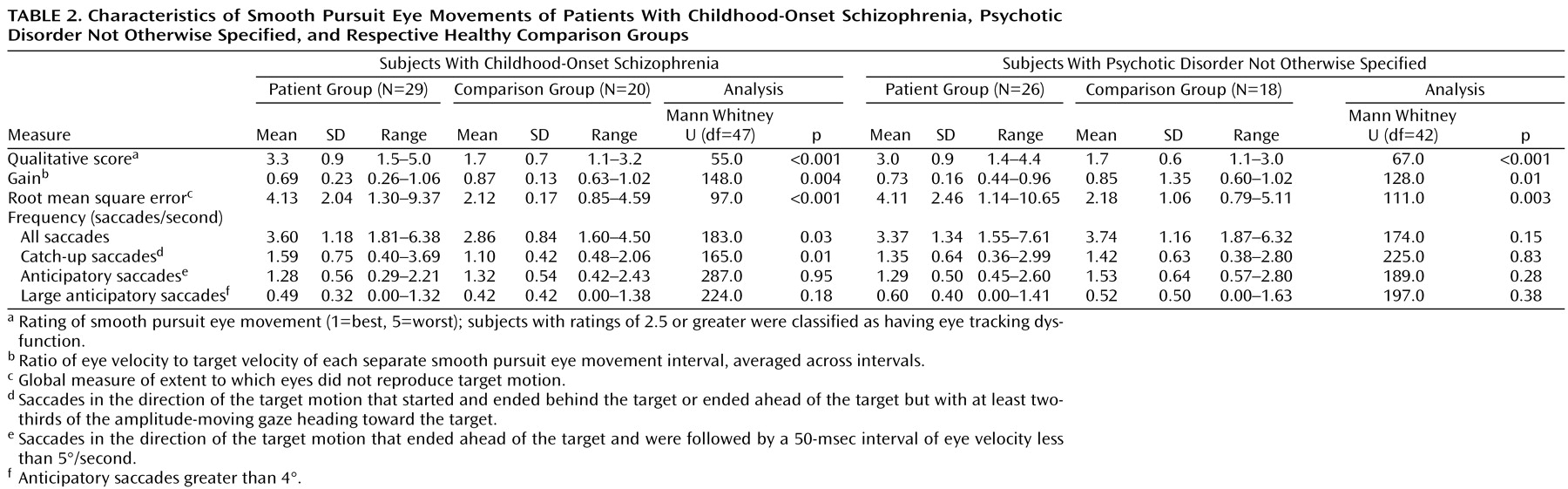
Significantly poorer qualitative ratings were seen among the childhood-onset schizophrenia patients than in the healthy volunteers (
Table 2). Significantly more subjects with childhood-onset schizophrenia (79.3%, N=23 of 29) than the healthy volunteers (20.0%, N=4 of 20) were found to have eye-tracking dysfunction (χ
2=14.3, df=1, p<0.01). The qualitative eye-tracking scores were also significantly worse in the patients with psychotic disorder not otherwise specified than in the healthy volunteers. Significantly more subjects with psychotic disorder not otherwise specified (65.4%, N=17 of 26) than healthy volunteers (11.1%, N=2 of 18) had eye-tracking dysfunction (χ
2=12.77, df=1, p<0.001).
For the 17°/second visual pursuit task, the subjects with childhood-onset schizophrenia and those with psychotic disorder not otherwise specified had significantly higher root mean square error than their respective healthy comparison groups. Significant differences in gain were also noted, with gain being lower in both patient groups (
Table 2).
After removing the 15 patients who were being treated with clozapine or a benzodiazepine, root mean square error remained significantly higher in the childhood-onset schizophrenia patients relative to the healthy comparison volunteers (U=37.0, df=32, p<0.001). Between-group significant differences for the other eye-tracking characteristics also held: gain (U=72.5, df=32, p<0.02), qualitative score (U=14.0, df=32, p<0.001), saccade rate (U=72.0, df=32, p<0.02), and rate of catch-up saccades (U=82.0, df=32, p<0.05). For the subjects with psychotic disorder not otherwise specified, after removing the two patients who were receiving lithium or carbamazepine, between-group significant differences in root mean square error, qualitative score, and gain remained (U=96.0, df=40, p=0.002; U=63.0, df=40, p<0.001; and U=111.2, df=40, p=0.008, respectively).
Relationship Between Eye Tracking, Age, and Clinical Measures
For the entire group of healthy comparison subjects (N=38), age was not significantly correlated with root mean square error (rs=–0.24, p=0.15), gain (rs=0.26, p=0.11), qualitative score (rs =–0.15, p=0.37), or rate of all saccades (rs=–0.12, p=0.49). There was a significant correlation between age and the rate of catch-up saccades (rs=–0.33, p=0.04). For both patient groups, after correction for multiple comparisons, there were no significant correlations between any eye-tracking measure and age, IQ, neuropsychological test performance, or severity of positive or negative symptoms.
Discussion
To our knowledge, this is the first comparative study of smooth pursuit eye tracking in pediatric patients with contrasting childhood-onset psychotic disorders. As expected, the childhood-onset schizophrenia patients exhibited a pattern of eye-tracking dysfunction characterized by poorer qualitative scores, lower gain, higher root mean square error, and a greater frequency of catch-up saccades than age-matched healthy volunteers. A similar pattern of eye-tracking dysfunction has been consistently reported in studies of adult schizophrenic patients unselected for age at onset
(11,
29,
34–
42). Recently, similar findings have also been reported (although with small anticipatory saccades) for a cohort of 10 childhood-onset schizophrenia subjects relative to healthy comparison subjects
(20). Together, these data support a hypothesis that childhood-onset schizophrenia is related to later-onset schizophrenia and thus may be a manifestation of the same disease process.
The main purpose of this study was to investigate whether the pattern of smooth pursuit eye tracking in subjects with psychotic disorder not otherwise specified resembled that in a group with narrowly defined childhood-onset schizophrenia. Patients with psychotic disorder not otherwise specified were found to have poor global tracking performance as marked by significantly worse qualitative ratings, higher root mean square error, and lower gain but no difference in frequency of catch-up saccades relative to healthy comparison subjects. These data along with a similar pattern of abnormalities observed in subjects with either psychotic disorder not otherwise specified or childhood-onset schizophrenia (prepsychotic linguistic deficits, family history, neuropsychological test performance, and quantitative brain MRI data) support a hypothesis that the two disorders may share some risk factors
(6).
Because much remains to be known about the pathophysiology of childhood-onset schizophrenia, it is difficult to fully define the relationship between psychotic disorder not otherwise specified and childhood-onset schizophrenia. Our initial clinical distinction, however, does appear to have prognostic importance. The 2- to 8-year clinical follow-up data indicate that psychotic disorder not otherwise specified does not progress onto schizophrenia but may instead develop into affective disorders
(7). Furthermore, this psychotic disorder not otherwise specified subgroup also differed from the childhood-onset schizophrenia group with respect to follow-up progression of brain MRI abnormalities. In childhood-onset schizophrenia, there is progressive loss of regional cortical gray during adolescence
(43,
44), whereas no significant loss of frontal or other regional gray volume was seen in the subjects with psychotic disorder not otherwise specified
(45). These data would argue for a separation of subjects with psychotic disorder not otherwise specified from future genetic analyses of childhood-onset schizophrenia.
It is theoretically possible that the pattern of neurobiological abnormalities that we observed in this psychotic disorder not otherwise specified subgroup might be seen in a broad range of children and adolescents with severe emotional disturbances. Other patient groups, such as adult bipolar patients
(17,
18), have been reported to have smooth pursuit tracking dysfunction. However, the biological relatives of bipolar patients have not been shown to have eye-tracking dysfunction
(11), suggesting that globally assessed eye-tracking dysfunction in affective disorders may be state-, rather than trait-, related
(46). Our preliminary data suggest that some measures of eye-tracking dysfunction are also seen in the biological first-degree relatives of childhood-onset schizophrenia patients
(47). Because eye tracking in younger children is harder to record and therefore has greater variability
(24,
48), the psychotic disorder not otherwise specified group is being followed longitudinally to establish the stability of the study findings. Also, family studies of the psychotic disorder not otherwise specified group are currently underway to address the question as to whether eye-tracking dysfunction in these patients may represent a state- or trait-related phenomenon.
Various lines of evidence suggest that the eye-tracking dysfunction of schizophrenia patients may arise from an underlying dysfunction of the prefrontal cortex. The identification of eye-tracking dysfunction in subjects with childhood-onset schizophrenia and their families may be of use for future genetic studies as a potential alternative phenotype for childhood-onset schizophrenia
(46). Such an alternative phenotype could increase the statistical power of genetic analyses in schizophrenic pedigrees by increasing the number of affected subjects. It is also possible that eye-tracking dysfunction may represent a biological deficit in schizophrenia that could be more closely related to a single gene effect than schizophrenia itself
(46).
Between 7 to 15 years of age, the performance of the smooth pursuit system continues to develop in healthy children
(24,
48,
49). As seen in previous studies, the correlation between age and smooth pursuit system variables accounts for at most 20% of the variance
(24), which may reflect differential rates of brain maturation in healthy children. In this study, age effects were dealt with by creating two healthy comparison groups matched in gender and age to each respective patient group.
Because children described as having “multiplex developmental disorder”
(5,
50) or borderline disorder
(4) bear some clinical resemblance to our psychotic disorder not otherwise specified group, cross-center replications of these findings and the inclusion of medication-naive patients will be necessary to define more homogeneous subgroups of patients.
The data from our study should be considered in light of other methodologic issues. The children with childhood-onset schizophrenia and psychotic disorder not otherwise specified both differed from their healthy comparison groups in terms of IQ and attentional disturbances. The discrepancy in IQ between patients and normal subjects may not be a confounding factor, since in normal children no consistent relationship between IQ and eye movement variables has been found
(24). Also, the lower IQ scores of the patients may not be reflective of their true abilities based on previous IQ testing
(8). Thus, we did not covary IQ (or its surrogate, vocabulary) in primary analyses.
A major shortcoming is that a monitor condition was not included in this protocol, which may have enhanced attention and provided a more conservative estimate of the subjects’ optimal performance
(15). In a study of children and adolescents, attention facilitation effectively normalized the high anticipatory saccade rate observed in the offspring of schizophrenic probands compared to community control subjects
(15). However the primary results of the study (i.e., global eye-tracking measure) remained unchanged such that children and adolescents who were offspring of depressed parents could not be distinguished from offspring of schizophrenic parents
(15). In the present study, it is unlikely that the eye-tracking dysfunctions in patients could be solely attributed to attentional disturbances. First, there was no significant correlation between gain or root mean square error with performance on tests of speeded visual-motor processing and attention (e.g., Trailmaking A and B and average of the coding and digit symbol subtests of the WISC-R/WAIS-R). Second, all patients were carefully supervised during the task to ensure that they were engaged in smooth pursuit and were instructed not to jump ahead of the target.
We chose to test patients while they were on stable medication regimens to enhance compliance and optimize performance
(51). The weight of evidence suggests that typical neuroleptics and antidepressants do not cause eye-tracking dysfunction in patients but that clozapine, sedatives, anticonvulsants, and other psychotropic medications (e.g., lithium carbonate) can influence eye-tracking performance
(10,
11,
52). Clozapine has been found to reduce gain scores and to significantly increase the frequency of catch-up saccades
(22). However, when we removed the subjects with childhood-onset schizophrenia receiving clozapine the findings of this study were unchanged.
In summary, given our evidence that subjects with psychotic disorder not otherwise specified do not progress onto schizophrenia but instead may develop affective disorders, these findings are comparable with at least some studies of eye tracking in bipolar disorder, although here results are inconsistent
(17,
18). The relative nonspecificity of these eye movement findings in children and adolescents and other differences in clinical and biological measures do support separation of subjects with psychotic disorder not otherwise specified from future genetic studies of early-onset schizophrenia.