Executive functions are those cognitive processes necessary for controlled information processing and coordinated actions
(1). Impaired executive functioning has served as the basis of a number of general theories of cognitive dysfunction in schizophrenia
(2–
6) and has been hypothesized to be related to the behavioral disorganization characteristic of this illness. With the growing awareness of the association between cognitive disability and poor outcome in schizophrenia (7), there has been a renewed interest in understanding the nature of impaired executive functioning in schizophrenia as well as its pathophysiological substrates.
Two broad sets of processes have been considered as contributors to executive functioning
(1). Strategic processes are those involved in the top-down control of cognition. These functions include representing and maintaining goals and allocating limited attentional resources. These functions appear to rely on the integrity of regions of the dorsolateral prefrontal cortex
(8,
9). A second set of processes, also essential for executive control, are those involved in the ongoing evaluation of performance. These evaluative functions are critical for the flexible adjustments of top-down control needed for a seamless adaptation to a constantly changing environment. A growing body of data implicate the anterior cingulate cortex on the medial surface of the frontal lobes in this function
(1,
10).
Much of the theoretical and empirical work to date related to impaired executive functioning has focused on impaired strategic processes and their association with impairments in the dorsolateral prefrontal cortex and related circuitry
(11–
14). These studies have provided insights into the nature of impaired top-down cognitive control in patients with schizophrenia and focused attention on the potential role alterations in the local circuitry of this region and its development
(15,
16) may have in cognitive disability. Relatively little attention has been paid to impaired performance monitoring functions in patients with schizophrenia. However, a number of studies that have used resting single photon emission computed tomography and positron emission tomography (PET)
(14,
17–
19) have suggested abnormalities of this region both at rest and during the performance of tasks engaging executive functions.
One way that the anterior cingulate cortex is thought to contribute to internal performance monitoring functions is through its sensitivity to errors. Event-related potential (10) and, more recently, event-related functional magnetic resonance imaging (fMRI) studies
(20,
21) have shown activation in this region associated with error commission as well as evidence that this activity is coupled with performance adjustments following error commission
(10). Preliminary electrophysiological evidence that error detection was impaired in patients with schizophrenia was reported by Kopp and Rist
(22), who reported that these patients exhibited diminished error-related negativity, a transient response locked event-related potential signal that is observed concurrent with making an error during a speeded response task. In the present study, we used event-related fMRI and a speeded reaction time task to test the hypothesis that a reduction in brain performance monitoring activity in the anterior cingulate cortex contributes to impaired executive functioning in patients with schizophrenia.
Results
To ensure that signal-to-noise ratios were not impaired by nonspecific factors such as differences in head movement during scanning or other nonspecific factors, estimated movement parameters were extracted from the automated image registration log files, and signal-to-noise ratio maps were computed. Six subjects (three schizophrenic and three comparison) were excluded from the analysis because they had mean movement in one or more dimension of more than 4 mm or degrees (approximately one voxel). For the remaining groups, there were no significant differences on any dimension on the estimated movement parameters. When the groups were contrasted on signal-to-noise ratio maps across all regions of interest for which group contrasts were conducted, no significant differences were observed (patients: mean=736, SD=737, comparison subjects: mean=704, SD=720).
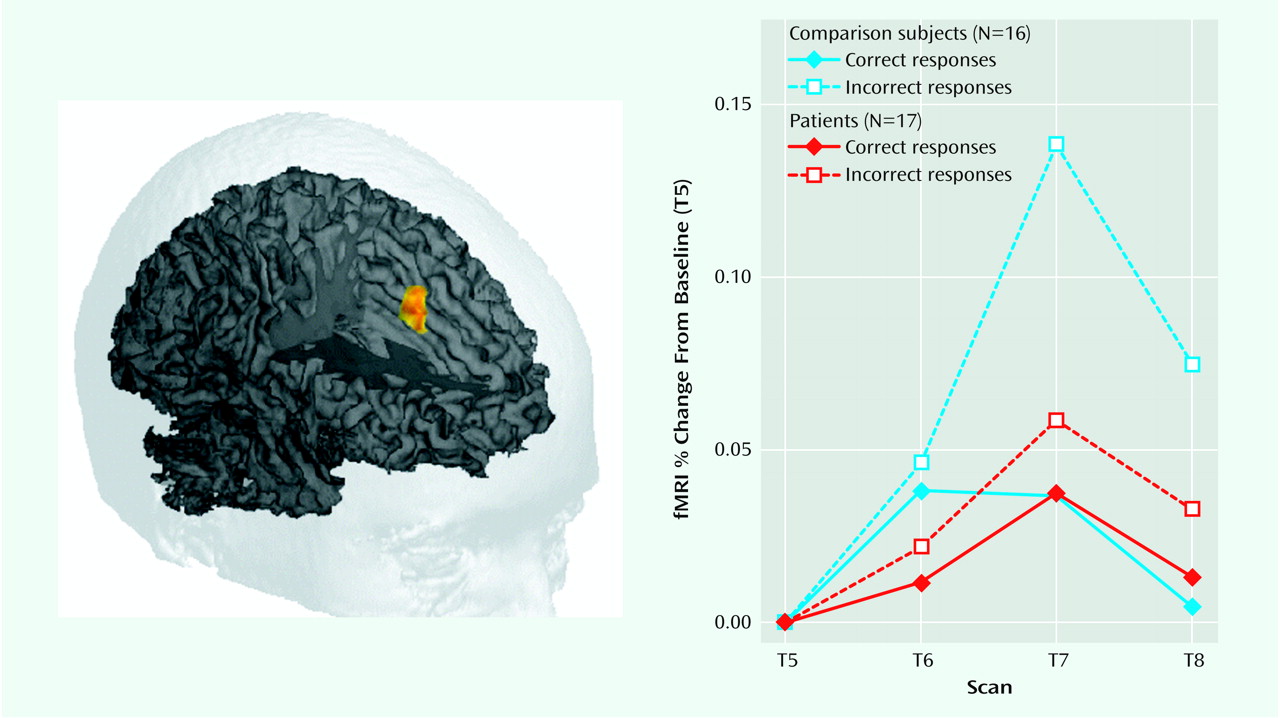
As in a previous study that used this paradigm (20), the healthy comparison subjects showed a robust, transient, response-related increase in the anterior cingulate cortex during error commission (75 voxels, centroid of activation [x, y, z]: 0, 27, 36). A small area of right anterior medial frontal cortex (Brodmann’s area 10) and left posterior parietal cortex (Brodmann’s area 40) also showed error-related activity in the healthy comparison subjects. Schizophrenic patients failed to show an increase in activity in the anterior cingulate cortex (or any other region of the brain) during commission of errors. In the direct contrast of the two groups, there was a large area of the anterior cingulate cortex, centered on the ventral bank of the cingulate sulcus (Brodmann’s area 24) and extending into adjacent Brodmann’s area 32, that showed a significant group-by-accuracy-by-scan interaction (54 voxels, centroid of activation: 2, 21, 36). The contrast between the error-related activity observed in the anterior cingulate cortex in comparison subjects and in the schizophrenic patients is shown in
>Figure 1.
When normal subjects make an error, their reaction times are slower on the subsequent trial
(29). This phenomenon, sometimes referred to as the “Rabbitt effect,” was considered to be evidence for central error monitoring processes long before cognitive neuroscience was able to show evidence of brain activity associated with such a process. It has previously been reported that the magnitude of the error-related negativity correlates with the degree of slowing following error commission
(10). We contrasted reaction times before and after commission of errors across the two groups and found that the patients with schizophrenia exhibited significantly less slowing of reaction time after error commission. For comparison subjects, the mean reaction after an error was 708.13 msec (SD=216.6), whereas after a correct response it was 596.7 msec (SD=172.3). For the schizophrenic patients, mean reaction times after incorrect and correct responses were 801.7 (SD=167) and 774.9 (SD=157.3) msec, respectively. Hence, the mean Rabbitt effect for the comparison subjects was 111.4 msec, whereas for the schizophrenia patients it was only 26.8 msec (F=6.1, df=1, 31, p<0.02).
Discussion
This is the first study to our knowledge to use event-related fMRI to investigate brain activity associated with the internal monitoring of performance in patients with schizophrenia. We found that relative to matched comparison subjects, error-related activity in the anterior cingulate cortex was impaired in the patient group. Associated with this impairment, error-related performance adjustments also differed between patients and comparison subjects, with patients exhibiting less of the normal reaction time adjustment following errors or Rabbitt effect. These results are consistent with our hypothesis that a performance-monitoring deficit based in the anterior cingulate cortex contributes to impaired executive functioning in patients with schizophrenia.
A number of previous investigators have proposed models of impaired self-monitoring in patients with schizophrenia
(30,
31). These models have proposed that the internal monitoring of actions is disrupted through an impairment of corollary discharge, the copy of a motor plan that is returned to a central monitor in order to evaluate a movement before or as it is executed. Some theories of error detection also assume an underlying mechanism involving a comparison of an efference copy of an action together with a representation of the correct action, referred to as the “comparator” hypothesis
(32). From this perspective, our results are consistent with a disturbance of an efference copy mechanism. However, previous theories related to altered efference copy in schizophrenia have focused on this disturbance as a potential mechanism for positive symptoms in which putatively self-generated actions are attributed to an external agent, e.g., inner speech is mistaken for an external voice. Our hypothesis of impaired performance monitoring in patients with schizophrenia emphasizes the relevance that this deficit may have for impaired executive functioning in this illness. Furthermore, we have proposed that error detection in humans can be understood without the need for a comparator function involving efference copy mechanisms
(33). On the basis of the observation that the same regions of the anterior cingulate cortex show activity during error commission and during tasks that elicit response conflict—as well as results from computational modeling studies that have shown that response conflict is particularly high during error commission—we have hypothesized that the anterior cingulate cortex contributes to evaluation of performance by detecting response conflict without the need for a comparator. In the present study, we could not examine activity related to response conflict because there were too few trials eliciting response conflict during the nondegraded blocks (generally fewer than five per subject). However, in a previous PET study that used the Stroop task
(34), schizophrenic patients exhibited lower anterior cingulate cortex activity during the color-incongruent, response conflict-eliciting condition of this task. Further studies examining both error-related and response conflict-related activity in the anterior cingulate cortex are currently underway in our laboratory.
As previously mentioned, executive functioning appears to be implemented in the brain by a distributed network of regions, including the dorsolateral prefrontal cortex, and it is highly likely that disturbances are present in this region of the brain in patients with schizophrenia. Could the changes in error-related activity observed in the anterior cingulate cortex in the present study simply be secondary to changes in other regions, such as the dorsolateral prefrontal cortex? We cannot exclude this possibility, but we believe that it is unlikely. In a study of the error-related negativity in subjects with focal lesions of the dorsolateral prefrontal cortex but intact anterior cingulate cortices, the frontal patients showed an increase in the event-related potential signal associated with correct response and no change with respect to the amplitude of the error-related wave form. The pattern of reduction of the error-related negativity in the study by Kopp and Rist
(22) and the pattern of MR signal change in the present study is quite different to that observed in the frontal patients. In these two studies there were no differences in activity associated with correct responses but markedly lower error-related effects. An examination of conflict-related activity in the anterior cingulate cortex may be even more informative with regard to this issue. An impairment in attentional control associated with dorsolateral prefrontal cortex dysfunction should elicit greater conflict. As such, impaired dorsolateral prefrontal cortex together with intact anterior cingulate cortex should produce greater cingulate response, while impaired conflict detection in this region would predict lower activity in this region. We have recently shown, using event-related methods, that top control functions of the dorsolateral prefrontal cortex and conflict-related activity in the anterior cingulate cortex can be dissociated in time
(9). With this approach it should be possible to tease apart the relative contribution of these two regions to impaired executive functioning in schizophrenia, and these studies are also underway in our laboratory.
A caveat regarding the results of the present study, as well as the previous event-related potential study
(22) suggesting diminished error-related brain activity in patients with schizophrenia, is that the patients were all treated with antipsychotic medications. Previous studies have shown that antipsychotic drugs decrease blood flow and metabolism in the anterior cingulate cortex. Reductions in anterior cingulate cortex resting metabolism and in the anterior cingulate cortex response during cognitive activation—which we would interpret as related to response conflict
(19)—have previously been reported in unmedicated patients, but it is unknown how antipsychotic drugs might affect error-related activity in this region of the brain. Further studies examining error-related and response conflict-related activity in the anterior cingulate cortex in unmedicated patients before and after the initiation of treatment are needed in order to examine the possibility that antipsychotic medication may have a deleterious effect on the internal monitoring of performance in schizophrenia. An additional limitation is that data from several subjects had to be excluded from analysis because of excessive movement or because they were unable to perform the task. This raises an issue regarding the generalizability of results obtained from functional brain imaging studies to the broad population of schizophrenic patients. While, on the one hand, this limitation implies that results from cognitive fMRI studies of schizophrenia might not always be fully generalizable to the broad population of patients, on the other hand it is likely that such studies are biased
against confirming hypotheses regarding the brain circuitry underlying impaired cognition in this illness, since the most severely affected patients cannot be studied. Hypotheses that are confirmed by these methods might then be considered to have survived a more stringent test by being confirmed in a subset of more mildly affected patients. The development of methods that permit more rapid data acquisition, as well as the use of simplified behavioral paradigms, may reduce the impact of this potential sampling bias in the future.
In conclusion, in the present study, patients with schizophrenia showed less error-related activity in the anterior cingulate cortex during event-related fMRI as well as associated impairments in performance adjustments following error commission. These data provide preliminary evidence for impaired anterior cingulate cortex-based evaluative function, and, hence, a specific contribution of disturbances in this region of the brain to impaired executive functioning in schizophrenia. Further studies examining response conflict-related activity during correct responses, medication effects, and the relative contributions of medial and lateral frontal cortical disturbances are likely to further increase our understanding of the functional neuroanatomy of cognitive disability in schizophrenia.