Posttraumatic stress disorder (PTSD) is a prevalent, often chronic, significantly disabling illness
(1,
2). PTSD is responsive to treatment with selective serotonin reuptake inhibitors (SSRIs)
(3,
4), but response rates rarely exceed 60%, and even fewer patients (20%–30%) experience improvement that could be characterized as remission. Consequently, a role exists for adjunctive treatments that might further improve outcomes.
Sleep disturbance is common among PTSD sufferers
(5,
6), often proving particularly resistant to treatment. Several open-label reports
(7–
9) suggest that olanzapine, an atypical antipsychotic agent with prominent sedating properties, may be useful in treating PTSD, especially (but not solely) helping reduce sleep disturbances. However, olanzapine monotherapy did not prove superior to placebo in a double-blind pilot study of 15 patients with PTSD
(10).
Combat exposure is known to result in a particularly virulent form of PTSD that tends to be chronic, disabling, and highly comorbid
(11). One study
(12) suggested that combat-related PTSD in veterans may be especially resistant to SSRI treatment, making this form of PTSD particularly apt for the testing of adjunctive therapies. To our knowledge, this is the first controlled trial to test the efficacy of an adjunctive pharmacological treatment for SSRI-resistant PTSD.
Method
Subjects were male patients from the VA San Diego Healthcare System with a clinically predominant diagnosis of DSM-IV PTSD (comorbid mood disorders were not exclusionary). The treating psychiatrist (M.B.S.) attempted to optimize their response to SSRI treatment by maximizing the dose, as tolerated. Patients whose PTSD symptoms were prospectively judged to be minimally responsive to 12 or more weeks (4 weeks or more at maximally tolerated doses) of treatment with an SSRI were eligible for the study. All subjects had chronic military-related PTSD; all but three were Vietnam veterans, with typical duration of illness in the range of 20–25 years. The presence of PTSD-related psychotic symptoms
(13,
14) was
not necessary for inclusion. All subjects provided informed written consent to participate in this study, which was approved by the University of California, San Diego, School of Medicine Human Research Protection Program.
Of the 21 patients enrolled in the study, two failed to return for their first medication assessment and were lost to follow-up. At the time of random assignment, of the remaining 19 patients (who are the subject of this report), five were taking fluoxetine (median dose: 40 mg/day), seven were taking paroxetine (median dose: 40 mg/day), and seven were taking sertraline (median dose: 200 mg/day). Subjects continued to take their maximally tolerated, stable dose of SSRI throughout the study and were randomly assigned to take either adjunctive olanzapine (10 mg) or placebo at bedtime for the first 2 weeks. This could then be increased to 20 mg at the next visit, if needed and tolerated. No other concurrent psychotropic medications were permitted.
Primary outcome measures were chosen to portray change in three symptom domains: posttraumatic stress (using the Clinician-Administered PTSD Scale for DSM-IV
[15]), depressive (using the self-rated Center for Epidemiologic Studies Depression Scale [CES-D Scale]
[16]), and sleep (using the self-report Pittsburgh Sleep Quality Index
[17]) symptoms. Changes in these measures were compared across treatments, as were the numbers of responders in each period that were defined by the Clinical Global Impression (CGI) scale of change as “much improved” or “very much improved” relative to the start of treatment. Last observed scores were carried forward wherever possible for noncompleters; Pittsburgh Sleep Quality Inventory scores were available only for completers. Two-tailed tests were used, with p<0.05 deemed statistically significant.
Results
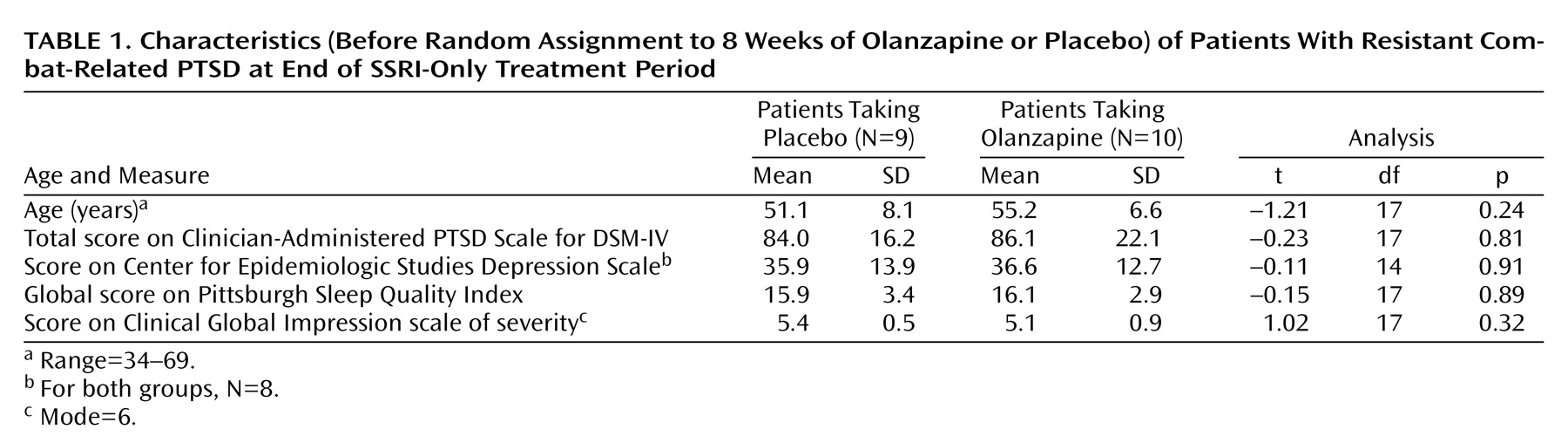
Mean scores at random assignment are shown in
Table 1. Patients had severe PTSD and depressive symptoms and pronounced sleep problems. Olanzapine was associated with a significantly greater reduction than placebo in PTSD symptoms, as measured by total score on the Clinician-Administered PTSD Scale for DSM-IV: mean=–14.80 (SD=14.16) versus mean=–2.67 (SD=10.55), respectively (t=–2.21, df=17, p<0.05). It was also associated with a significantly greater reduction than placebo in sleep disturbance, as measured by the global score on the Pittsburgh Sleep Quality Index: mean=–3.29 (SD=3.15) versus mean=1.57 (SD=2.76) (t=–3.07, df=12, p=0.01). Change in Pittsburgh Sleep Quality Inventory score was moderately correlated with change in Clinician-Administered PTSD Scale for DSM-IV score (r=0.66, df=15, p=0.01), suggesting that enhanced sleep accounted for much of the reported improvement.
Olanzapine was also associated with a significantly greater reduction than placebo in depressive symptoms, as measured by the CES-D Scale: mean=–5.25 (SD=6.27) versus mean=4.88 (SD=9.66) (t=–2.49, df=14, p<0.03). The two treatments did not significantly differ in the percentage of subjects deemed responders on the CGI scale of change: three of 10 (30%) taking olanzapine versus one of nine (11%) taking placebo (p=0.58, Fisher’s exact test).
The mean dose of olanzapine was 15.00 mg/day (SD=5.25) compared to the mean dose of placebo, which was 20.00 mg/day (SD=0.00) (t=2.83, df=17, p<0.02). Weight gain was significantly greater with olanzapine than placebo: mean=13.2 lb (SD=5.9) versus mean=–3.0 lb (SD=6.5) (t=4.32, df=11, p=0.001). Early protocol terminations occurred for three patients taking olanzapine (two for somnolence; one for unspecified reasons) and for two patients taking placebo (both for lack of efficacy).
Discussion
We found the atypical antipsychotic olanzapine to be superior to placebo as an adjunct in the treatment of SSRI-resistant PTSD. Beneficial effects on sleep and depressive symptoms, in particular, were noted. Still, when global clinical improvement was considered, response rates to adjunctive olanzapine were fairly low (30%) and not statistically superior to placebo (11%). This suggests that although improvement in symptoms was attained with olanzapine, the overall magnitude of effects was modest for most patients (but clinically meaningful for some). Given the typical chronicity and severity of combat-related PTSD, even small gains (such as those seen in this study) must be cherished.
There is a subgroup of combat-related PTSD patients with psychotic symptoms
(13,
14). These patients seem to have a more severe variant of PTSD and may benefit from the addition of antipsychotic agents to their treatment regimens. None of the patients in our study had psychotic symptoms, yet olanzapine was still helpful for some. This observation fits with emerging data in which olanzapine has been noted to successfully augment SSRI effects in two other conditions, even in the absence of psychotic symptoms: obsessive-compulsive disorder and major depressive disorder
(18,
19).
Mean weight gain in subjects receiving olanzapine was approximately 13 lb. The benefits of using this antipsychotic agent in conjunction with an SSRI for treating PTSD must be balanced against the potential detrimental health effects associated with this magnitude of weight gain. Effects on glucose regulation should also be systematically evaluated
(20).
We cannot rule out the possibility that more prolonged treatment with an SSRI would have resulted in additional improvement. Indeed, in one study in which sertraline treatment was continued up to 36 weeks
(21), 20%–25% of the improvement in scores on the Clinician-Administered PTSD Scale for DSM-IV occurred between weeks 12 and 36. Practically, however, it is doubtful that most patients (or clinicians) would be willing to persist with a treatment that had negligible effects after 12 weeks in the hope that additional gains would accrue with prolonged treatment. There thus remains a clear need for interventions that can potentiate the effects of SSRIs for PTSD. Pharmacological (including olanzapine and other atypical antipsychotic agents) and psychological therapies should be systematically tested for this purpose under controlled conditions.