Changes in nerve growth factor concentrations in the course of several diseases (alcoholic and diabetic neuropathy, Alzheimer’s disease, lesion models of the cholinergic basal forebrain) indicate that endogenous nerve growth factor concentrations follow a distinctive temporal pattern (for review see reference
1). Specifically, an initial decrease of nerve growth factor at the onset of pathological processes has been observed in a postmortem study of Alzheimer’s disease
(2), which found that nerve growth factor levels were lower than normal in the frontal cortex of patients who were not demented but did have senile plaques. Within the framework of the “amyloid cascade hypothesis”
(3), these patients may be considered as having preclinical Alzheimer’s disease or at risk for Alzheimer’s disease. Because no similar pattern was noticed in nondemented patients without senile plaques in the frontal cortex, this may point to a nerve growth factor deficiency in patients at a preclinical stage of Alzheimer’s disease
(2). Thus, nerve growth factor may be reduced at the onset of neurodegenerative processes for reasons still unknown. This reduction is followed by an increase in nerve growth factor in most pathological conditions, which could be understood as an attempt at counterregulation to maintain adequate nerve growth factor levels in endangered neurons that are dependent on nerve growth factor
(1,
4).
There are several cellular sources of nerve growth factor within and outside the CNS
(1), and there is experimental evidence
(5) for a bidirectional transport between both compartments. Moreover, nerve growth factor acts as a systemic or humoral cytokine in addition to its neurotrophic properties
(6). If the blood-brain barrier function is altered in terms of increased permeability in the early stages of Alzheimer’s disease
(7,
8), nerve growth factor changes within the CNS might be paralleled by serum levels, which then could be used to examine the hypothesis of a transient decline of nerve growth factor in the course of Alzheimer’s disease. Therefore, we expected lower levels of serum nerve growth factor in subjects who later developed Alzheimer’s disease than in those who were not affected cognitively and subjects already suffering from Alzheimer’s disease.
Method
Subjects were recruited from two sources: the Berlin Aging Study
(9) and the Berlin Memory Clinic, an outpatient memory clinic. All members of the comparison group (N=17) came from the Berlin Aging Study, a population-based multidisciplinary study of aging. Subjects in the predementia group (N=17) and in the Alzheimer’s disease group (N=17) were recruited from the Berlin Aging Study (N=10 in each group) and the Berlin Memory Clinic (N=7 in each group). Among 516 Berlin Aging Study volunteers, 313 were still alive after 3 years; 206 of these volunteers participated in the follow-up examination. Fifteen incident cases of Alzheimer’s disease were detected, and serum from the baseline examination was available for 10 of these subjects. Subjects from the Berlin Memory Clinic were repeatedly seen and included in this study when they received a diagnosis of Alzheimer’s disease (N=7).
Although the instruments for primary data collection differed somewhat between the Berlin Aging Study and the Berlin Memory Clinic, dementia diagnoses were made according to DSM-III-R and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria for probable Alzheimer’s disease
(10) in both facilities, with the exception of the age at onset criterion. Predementia was defined as not fulfilling any dementia criterion at baseline examination but as being diagnosed as demented on the follow-up examination, which occurred 3 years later on average. Participants also had to be free of somatic diseases that might influence serum nerve growth factor concentration (i.e., polyneuropathy, cancer, and other consuming disorders). Subjects in the three groups were matched for sex and age (analysis of variance [ANOVA]: F=0.13, df=2, 48, p=0.88). Written informed consent was obtained from each subject who participated in the study.
Nerve growth factor levels were measured in serum aliquots drawn before breakfast during the baseline examination and stored at –70°C for batch processing. Serum nerve growth factor was quantified by a modification of a highly sensitive and specific fluorometric two-site immunoassay, described elsewhere
(2). The nerve growth factor detection limit was set as 0.50 pg/ml. Differences between groups were analyzed with log-10-transformed nerve growth factor levels by Kruskal-Wallis H test and Mann-Whitney U tests for individual group comparisons and with ANOVA. All p values are two-tailed.
Results
Mini-Mental Health Examination scores differed among the groups. The comparison subjects had the best scores (mean=27.9, SD=1.7); the predementia group had intermediate scores (mean=25.4, SD=3.2); and the Alzheimer’s disease group had the lowest scores (mean=19.3, SD=5.3) (F=22.5, df=2, 48, p<0.001).
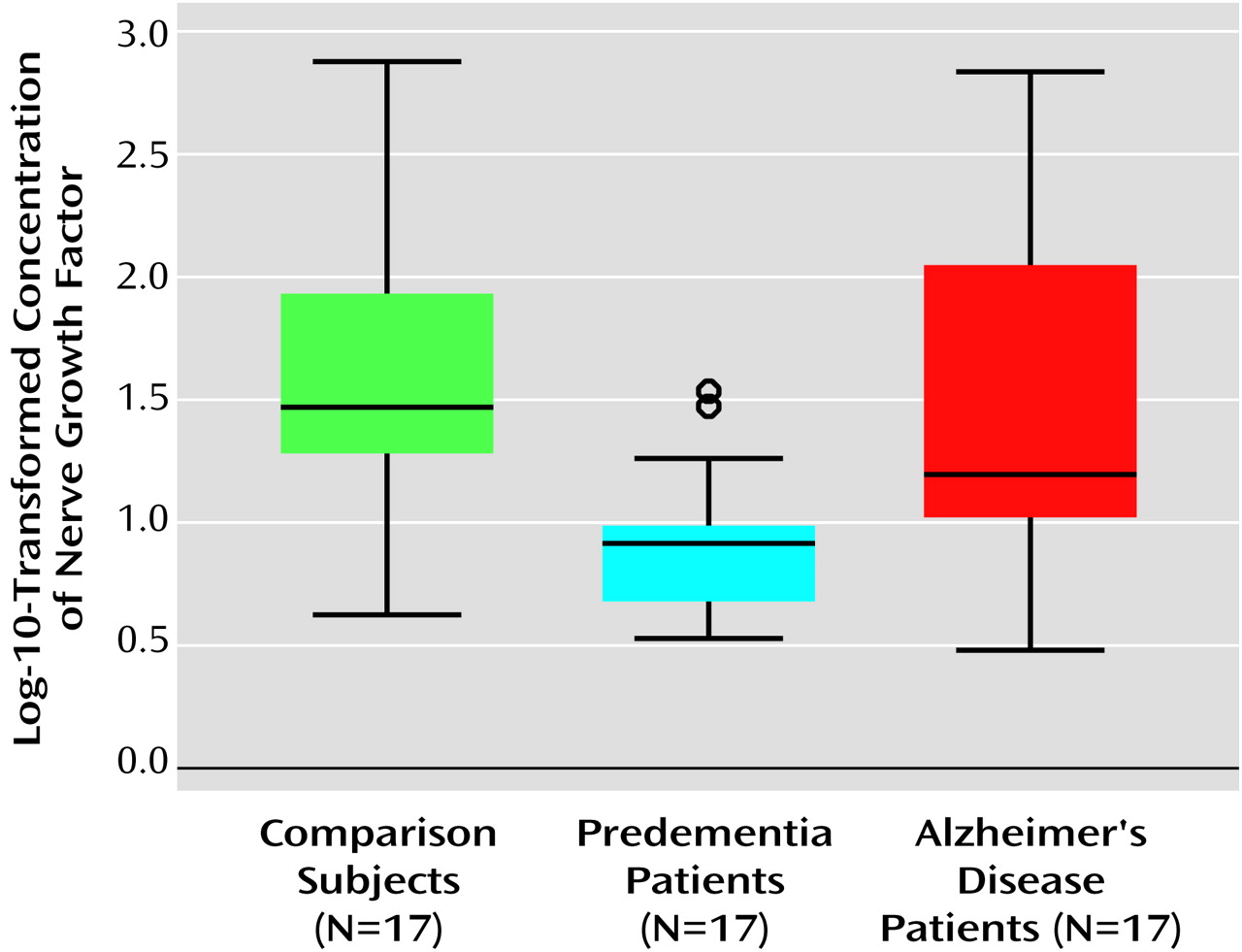
Nerve growth factor levels (
Figure 1) differed as follows: the comparison subjects had the highest mean level (mean=1.62, median=1.47, SD=0.59, interquartile range=0.74), the predementia group had the lowest mean level (mean=0.91, median=0.91, SD=0.30, interquartile range=0.40), and the Alzheimer’s disease group had a slightly lower mean level than the comparison subjects (mean=1.44, median=1.19; SD=0.65, interquartile range=1.09). Results of Kruskal-Wallis H test yielded χ
2=15.32, df=2, p<0.001; pairwise group comparisons revealed significant differences between the predementia group and the comparison group (Mann-Whitney U=37, p<0.001) and between the predementia group and the Alzheimer’s disease group (Mann-Whitney U=65, p=0.005). ANOVA results also were significant (F=7.87, df=2, 48, p<0.001); post hoc comparisons (Tamhane’s T2) showed differences between predementia subjects and comparison subjects (p<0.001) and between the predementia group and the Alzheimer’s disease group (p=0.02).
Discussion
This case-control study evaluated the relationship between nerve growth factor concentration and subsequent development of Alzheimer’s disease. Several reports examined nerve growth factor content in Alzheimer’s disease within the CNS
(4), but to our knowledge no study exists that compares nerve growth factor serum levels in cognitively intact subjects, subjects who later developed Alzheimer’s disease, and subjects already suffering from this condition (i.e., no study analyzed the temporal course of nerve growth factor during development of Alzheimer’s disease). In the current study we found that serum nerve growth factor concentration was lower in subjects who later developed Alzheimer’s disease than in healthy comparison subjects and subjects who already had Alzheimer’s disease. The fact that distributions of serum levels overlapped prevents clinical application at present. However, our finding supports the hypothesis of reduced nerve growth factor availability within the CNS at the time of onset of Alzheimer’s disease (2, 4) and the later tendency for nerve growth factor serum concentrations to “normalize”
(1,
11), although regulatory, cellular, and transfer mechanisms across the blood-brain barrier remain to be investigated.
If our results can be confirmed, serum nerve growth factor may become a candidate for a biomarker of early, clinically silent Alzheimer’s disease. Recognizing problems of the early diagnosis of Alzheimer’s disease
(12) and its enormous consequences, we feel that additional markers could enhance the reliability of early diagnosis in the future and thus improve the usefulness of diagnostic concepts like mild cognitive impairment that are empirically uncertain
(13).
This study has certain limitations: peripheral nerve growth factor content was examined but not central nerve growth factor content, and subjects were recruited from two sources, which might result in greater heterogeneity. However, all subjects with dementia were diagnosed according to the same standard (DSM-III-R and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria for probable Alzheimer’s disease). Moreover, the case-control design implies that we did not take repeated nerve growth factor measures but compared matched subjects at different stages of Alzheimer’s disease with unaffected comparison subjects. Therefore, a longitudinal strategy based on repeated nerve growth factor measures should be pursued to corroborate the potential of nerve growth factor as a biomarker for Alzheimer’s disease, preferably in future studies of mild cognitive impairment.