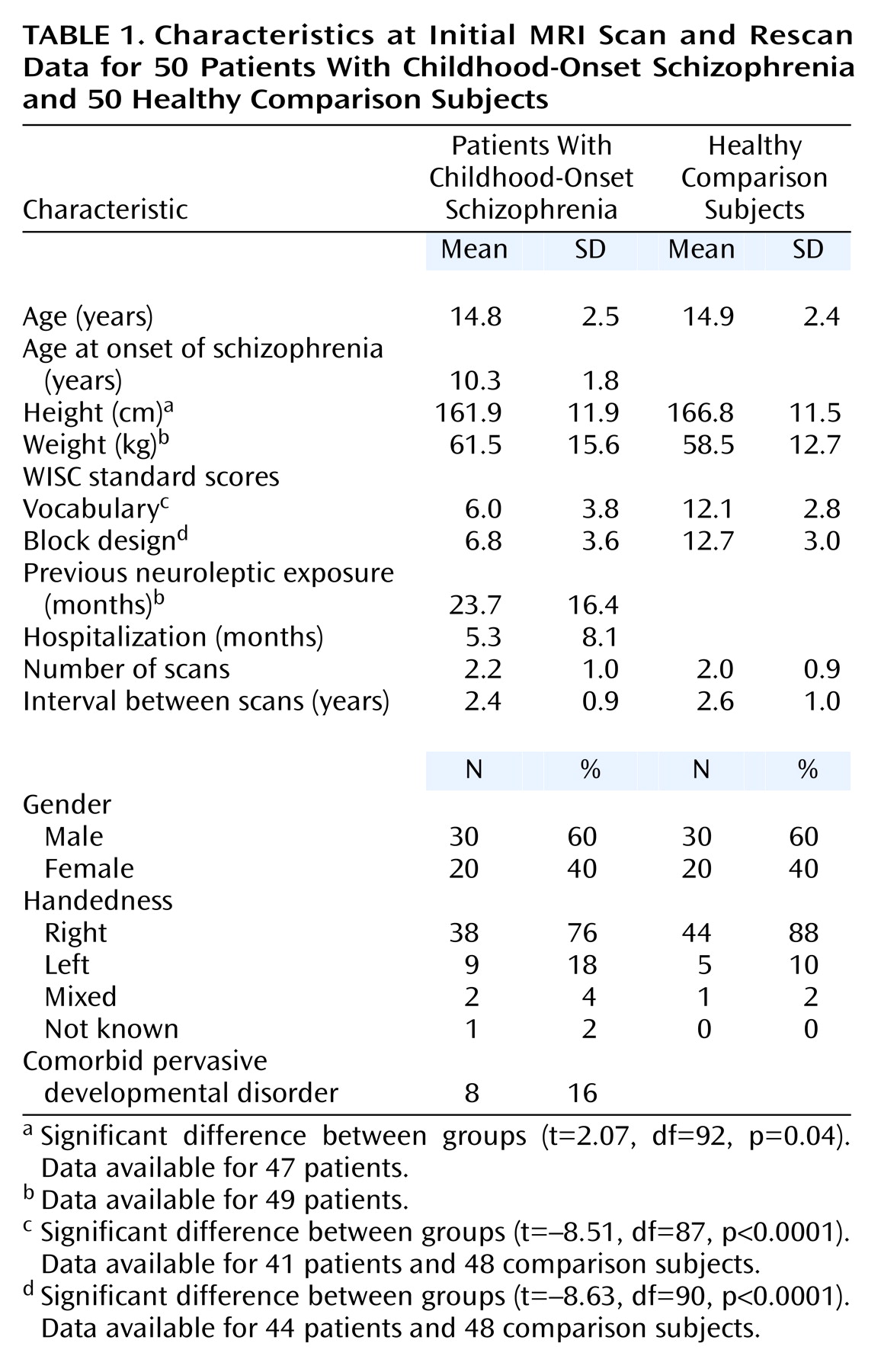
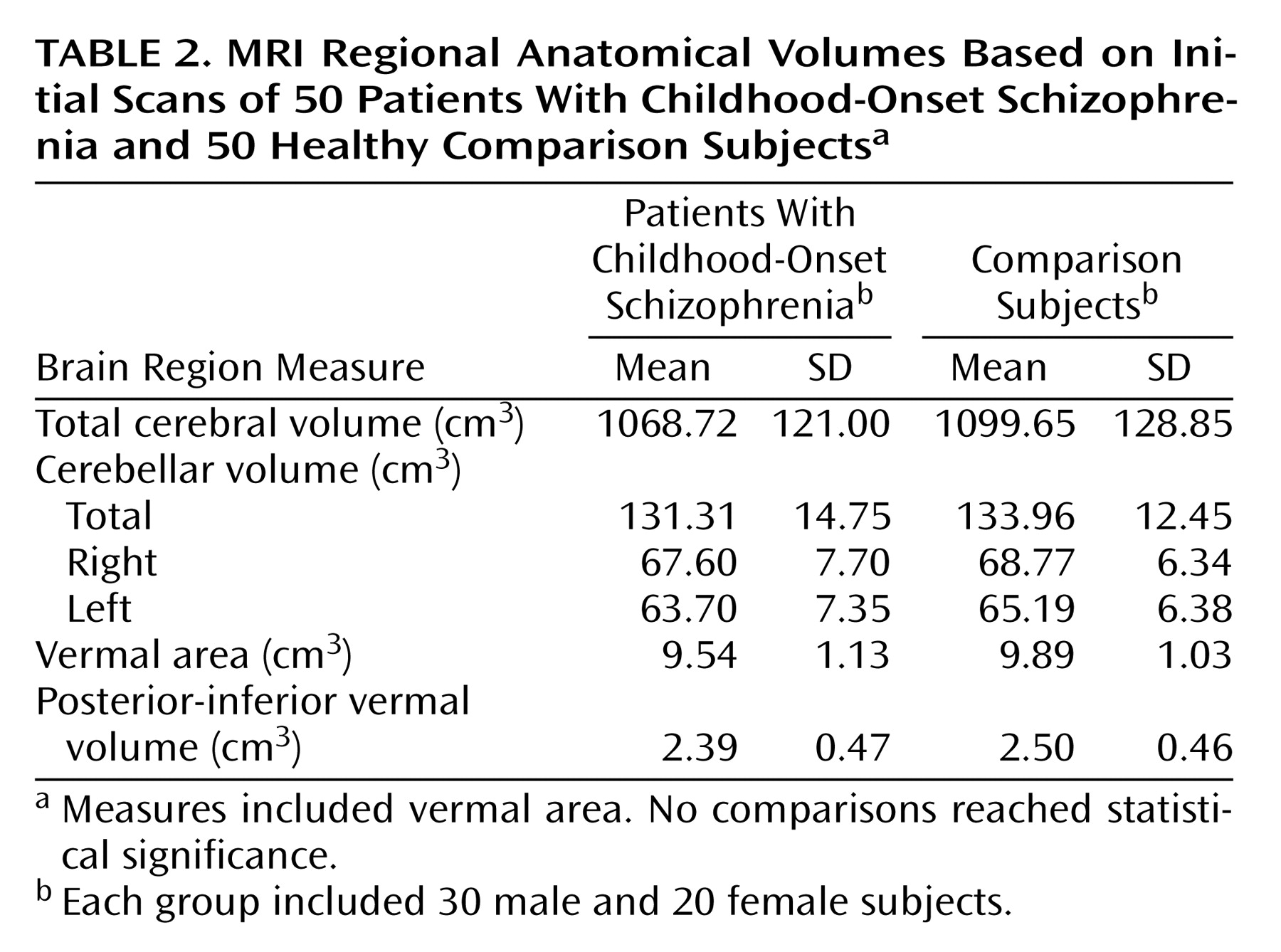
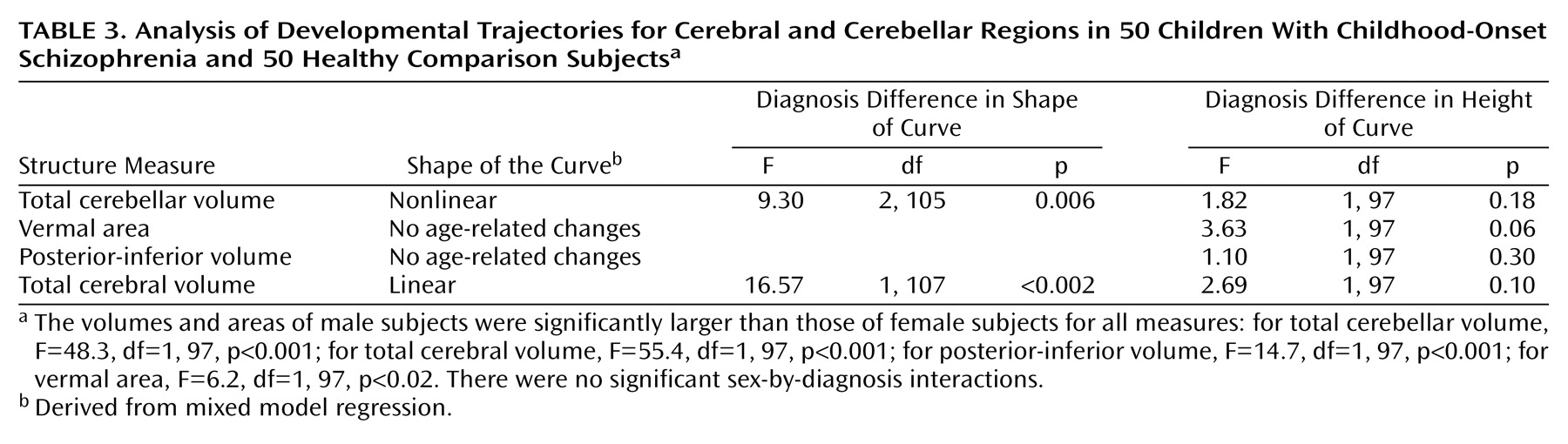
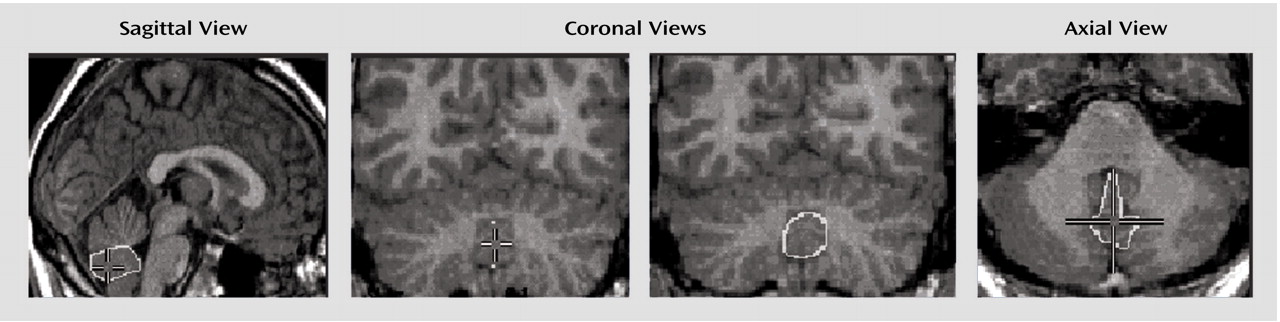
Childhood-onset schizophrenia, defined as onset of psychosis by age 12, is clinically and neurobiologically continuous with later-onset schizophrenia
(1). Patients with this rare, severe illness have profound impairment in premorbid development
(2) and resemble adult patients with poor-outcome schizophrenia. Previous National Institute of Mental Health (NIMH) brain imaging studies of childhood-onset schizophrenia reported progressive decreases in total volume in the cerebrum, hippocampus
(3), and frontal and temporal cortical gray matter
(4).
Cerebellar abnormalities have been reported, albeit inconsistently, in adult patients with schizophrenia. Some postmortem studies found smaller anterior vermal lobe with Purkinje cell dropout and thinning of granular and molecular layers
(5) as well as reduction in linear density of vermal Purkinje cells
(6), although others found no abnormalities
(7,
8). Similarly, early computed tomography studies reported cerebellar atrophy
(9,
10) and smaller cerebellar vermis
(11–
13), but quantitative magnetic resonance imaging (MRI) studies have produced inconsistent results (reviewed in references
14 and
15). Positive findings include reduced hemisphere and vermal volumes
(16–
18) and larger vermal white matter volume
(19), but other MRI studies have shown no significant differences between patients with schizophrenia and comparison subjects
(20–
23). Studies of midsagittal vermal area in schizophrenia found larger area of lobules VI–VII
(24), smaller anterior vermal lobe and smaller total vermis area
(25,
26), or no abnormalities
(27–
29). Finally, one prospective MRI study reported significantly reduced right cerebellar volume in schizophrenia
(30). Differences in subject selection, image acquisition, image analysis, and definition of boundaries make comparisons across studies difficult.
In a previous cross-sectional MRI study of 24 patients with childhood-onset schizophrenia, we found reduced vermal midsagittal area and posterior-inferior lobe volume
(31). A subset of unmedicated adolescents with childhood-onset schizophrenia performing an auditory continuous performance task during positron emission tomography had greater than normal cerebellar glucose metabolism
(32). Because prospective MRI studies are more sensitive to developmental change
(33), the present study examined prospective anatomical brain scans during adolescence. Total cerebellar and posterior-inferior vermal volumes and the vermal midsagittal area were measured in patients with childhood-onset schizophrenia and healthy volunteers. We hypothesized that a decrement in cerebellar and posterior-inferior vermal volumes would be seen throughout adolescence. We were also interested in whether cerebellar changes paralleled previously reported cortical gray matter loss for this group.
Method
Childhood-Onset Schizophrenia
Children and adolescents were recruited nationally for an ongoing study of childhood-onset schizophrenia
(34). Inclusion criteria were a DSM-III-R or DSM-IV diagnosis of schizophrenia with onset of psychosis before age 12, a premorbid full-scale IQ of at least 70, the absence of active medical or neurological disease, and poor response to or inability to tolerate treatment with at least two different neuroleptics. The diagnosis was established by using previous records and clinical and structured interviews of the children and parents as described in detail elsewhere
(35).
The study group of 50 patients with childhood-onset schizophrenia for whom at least one scan was available included 20 females. At baseline and at each follow-up visit, subjects were administered the WISC-R, the WISC-III, or the WAIS-R as appropriate. At follow-up, WISC raw scores for information and comprehension subscales were obtained to compare performance on these scales independent of subject age. A previously described method
(2) was used to review premorbid neuropsychological, school, and medical records. Twenty-seven patients (nine of whom were female) had developmental language difficulties, and nine had comorbid pervasive developmental disorder, not otherwise specified, according to the Autism Screening Questionnaire
(36).
Analyses of initial scans included 50 scans per diagnostic group. Patients returned for rescan approximately every 2 years. In the childhood-onset schizophrenia group, 19 patients had two, 12 had three, and five had four scans. In the comparison group, 19 subjects had two, 13 had three, and two had four scans. Developmental analyses were based on 108 childhood-onset schizophrenia scans and 101 comparison group scans. Using only subjects with more than one scan, we examined the relationship between cerebellar volume change and clinical or anatomical measures for 34 healthy volunteers and 36 patients with childhood-onset schizophrenia. All patients with childhood-onset schizophrenia were taking neuroleptics at follow-up, most frequently a combination of clozapine and at least one other antipsychotic medication (N=14), clozapine alone (N=12), or antipsychotic drugs other than clozapine (N=10).
Healthy Volunteers
Fifty healthy children and adolescents matched for age, sex, and handedness were recruited from the community (
Table 1). Structured rating scales and interviews of child and parents were performed as described elsewhere
(37).
Assent from the child and written consent from the parents were obtained for both patients and comparison subjects. The NIMH Institutional Review Board approved this study.
MRI Image Acquisition
All subjects were scanned on the same GE 1.5-T Signa scanner (GE Medical Systems, Milwaukee). T
1-weighted images with contiguous 1.5-mm slices in the axial plane and 2.0-mm slices in the coronal plane were obtained by using three-dimensional spoiled gradient recalled echo in the steady state. Imaging parameters were echo time=5 msec, repetition time=24 msec, flip angle=45°, acquisition matrix=192×256, number of excitations=1, and field of view=24 cm. Head placement was standardized as previously described
(3).
Image Analysis
Cerebrum and cerebellum
Total cerebral and cerebellar volumes were quantified by using a three-part fully automated image analysis process described in detail elsewhere
(33,
38). Reliability of the algorithms equaled unity for test-retest with the same scan. Intraclass correlation coefficients (ICC) for 10 subjects rescanned after leaving and reentering the magnet exceeded 0.98 for both measures. Automated and hand-traced measures of cerebellar volume were highly reliable (N=17, ICC=0.94).
Vermis
Midsagittal vermal area and posterior-inferior vermal lobe volume, excluding the cerebellar tonsils, were hand-measured by one rater (A.C.V.) using the MNI Display program (Montreal Neurological Institute, McGill University, Montreal) on a Silicon Graphics workstation (Mountain View, Calif.). Beginning with the cerebral midsagittal slice, the best vermis midsagittal slice, which differed from the midcerebral 63% of the time
(39), was selected in sagittal view and confirmed in coronal and axial views (
Figure 1). Areas for anterior, superior-inferior, and posterior-inferior lobes were hand-traced on the vermis midsagittal slice and added to derive total vermis area. Boundaries for the anterior lobe are the primary fissure and fourth ventricle; boundaries for the superior-inferior lobe were the primary and prepyramidal fissures; boundaries for the posterior-inferior lobe (lobules VIII–X) were the prepyramidal fissure and fourth ventricle
(31,
40).
Posterior-inferior volume was obtained by starting with the midsagittal outline that provided superior and inferior landmarks on each coronal slice. Lateral boundaries for each coronal slice were the CSF-gray matter interface (
Figure 1), and filled coronal areas were checked slice by slice in axial and sagittal views. Measurement of volumes of anterior and superior-anterior vermis was not attempted because these regions do not have true lateral boundaries
(40).
Interrater reliability between primary (A.C.V.) and secondary (A.K.) raters was obtained twice to check for rater drift with at least 10 brains per run; mean ICC=0.94 (SD=0.06) for posterior-inferior volume, and mean ICC=0. 93 (SD=0.06) for vermal area. Intrarater reliability was verified on three occasions: mean ICC=0.87 (SD=0.07) for posterior-inferior volume, and mean ICC=0.93 (SD=0.02) for vermal area.
Statistical Analysis
Fisher’s exact and Mann-Whitney test procedures were used to evaluate the comparability of patient and comparison groups.
Polynomial growth models were used to examine growth patterns of brain structures for initial (cross-sectional) scans. The initial cubic model was size=intercept + beta1* (age–mean age) + beta2* (age–mean age)2 + beta3* (age–mean age)3 + ε.
The model parameters (intercept and beta coefficients) were initially allowed to vary by sex and diagnostic group. The full cubic model was compared with simpler quadratic, linear, and constant models. Once the order of the model was established, testing was performed to determine whether an additive model could replace the interactions between sex and diagnostic group for the height and shape parameters of the curves. Hypothesis tests and model selection were initially based on F statistics. For F statistics with p values less than 0.10 that related to group or sex differences, permutation tests were performed to lessen the likelihood that a significant finding was due to a small number of outliers. To implement this procedure, 500 analyses using the preferred model were performed in which the sex and diagnostic group designation were randomly reassigned. The original F statistic based on the correct group and sex designation was compared with the resulting 500 F statistics, and an empirical p value was obtained.
The same polynomial growth models were used for the analysis of longitudinal data including all of each individual’s scans
(41,
42). To account for within-person correlation, intercepts were treated as normally distributed random effects that varied by individual, while beta coefficients for age, age-squared, and age-cubed terms were modeled as fixed effects. Although the statistical information provided by individuals with only one scan may be less than that obtained from those with multiple scans, single scans do provide additional information about between-person variation and overall curve shape.
Spearman correlation was used to examine the relationship between slopes for cerebellar and total cerebral volume loss. Slopes were calculated only for subjects with more than one scan; for those with more than two scans, first and last scans were selected. Stepwise regression and analysis of variance were used to examine clinical and treatment variables in relation to cerebellar volume decrease in childhood-onset schizophrenia.
Discussion
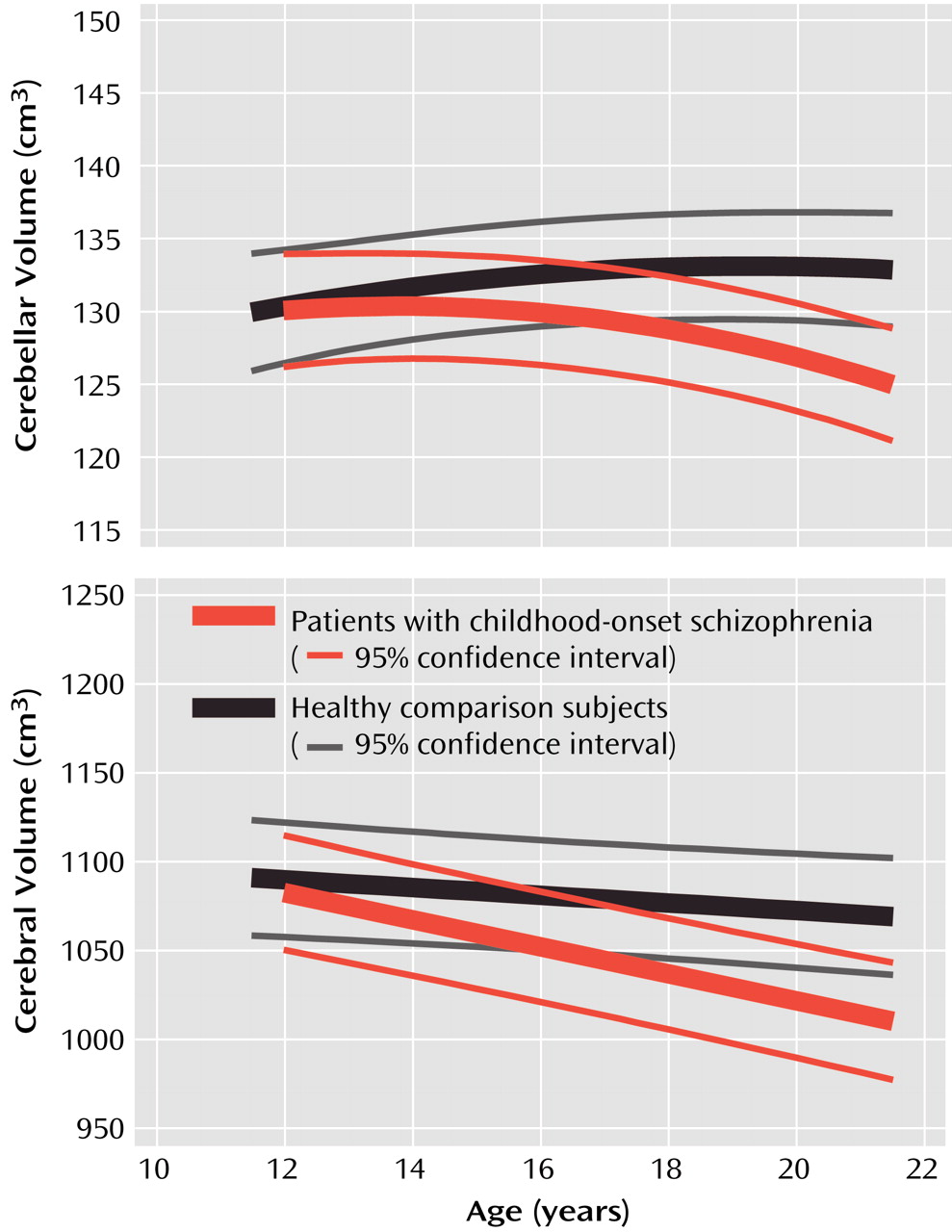
In this prospective study of cerebellar morphology in childhood-onset schizophrenia, a progressive loss of cerebellar volume in childhood-onset schizophrenia was found during adolescence, paralleling the loss in total cerebral volume previously reported for this group
(3). The cerebellum is one of the first brain structures to begin to differentiate and one of the last to achieve maturity
(43), reaching peak volume several years later than total cerebral volume (Giedd et al., unpublished data). However, in patients with childhood-onset schizophrenia, cerebellar loss seems to start at about the same age (within 1 year, by visual examination of confidence intervals) as loss of cerebral volume (
Figure 2). The fact that cerebellar tissue loss appears simultaneously with that of the total cerebrum suggests a generalized abnormal process in childhood-onset schizophrenia superimposed on normal development.
When only initial scans were examined, no difference in cerebellar volume was seen between the 50 patients with childhood-onset schizophrenia and the 50 normal comparison subjects. As with other measures in patients with childhood-onset schizophrenia and healthy subjects, only the availability of prospective longitudinal data revealed subtle differences in developmental trajectories
(3,
33) that were not detectable in cross-sectional studies with the same subjects
(37,
44).
These findings are consistent with reported abnormal cerebellar function in childhood and adult schizophrenia
(32,
45–47). There is accumulating evidence for a cognitive role for the cerebellum (see review in reference
48), including executive function and working memory, which are impaired in schizophrenia
(49–
53). Positron emission tomography studies have revealed abnormalities in cerebellar blood flow while patients carry out cognitive tasks
(46,
47). A model of schizophrenia as secondary to disrupted development in a cortico-cerebellar-thalamic-cortical circuit
(46) has been termed “cognitive dysmetria”
(54,
55), referring to incoordination in the processing, prioritization, retrieval, and expression of information. Unlike a previous study
(56), however, we found no significant relationship between any symptom pattern or change in any clinical or cognitive measure and our measures of cerebellar volume loss. We did not have available measures of motor function, which might have revealed a relationship to cerebellar volume loss.
We did not replicate previous findings of decreased midsagittal vermal area and posterior-inferior vermal lobe volume
(31), possibly due to the vagaries of hand measurements of small structures. In spite of good intrarater and interrater reliabilities, the anatomy of the vermis is difficult to delineate
(40), rater measures are subject to drift, and techniques for hand-measuring are fraught with methodological issues such as location of vermal midslice
(39,
57,
58).
Limitations of the study include substantially lower IQ for the patient group. Moreover, since all of our patients were medicated, we cannot rule out the possibility that our findings reflect the effects of neuroleptic medications. Our focus on treatment-resistant subjects also limits the generalizability of these findings.
In summary, the excess loss of brain tissue during adolescence seen in this and other studies may be a trait marker for schizophrenia. Since there is evidence of reduction of cerebellar volume in relatives of adult patients with schizophrenia compared with control subjects
(59), we are currently conducting a prospective brain MRI study of the siblings of our patients.