Previous research indicates that verbal learning and memory deficits are among the most severe cognitive deficits observed in schizophrenia patients
(1,
2). In a previous functional magnetic resonance imaging (fMRI) study of outpatients with remitted schizophrenia, we found attenuated frontotemporal activation during episodic encoding and recognition of words relative to healthy subjects
(3). However, it needs to be considered that all patients investigated in this study were treated with second-generation antipsychotics, which are believed to have beneficial effects on cognitive functioning
(4). Furthermore, only patients who had been clinically stable for a period of at least 6 months were included in the study. To address these limitations, we investigated activation patterns in unmedicated patients during an acute episode of schizophrenia.
Despite neuropsychological evidence that schizophrenia patients are impaired in the encoding of verbal information
(5), we hypothesized that recognition memory for words would be preserved
(6). Given previous neuroimaging research
(7,
8), we further hypothesized that unmedicated patients with schizophrenia would fail to engage dorsolateral prefrontal and limbic/paralimbic regions during the performance of a verbal memory task and that they would instead recruit nonspecialized brain systems.
Results
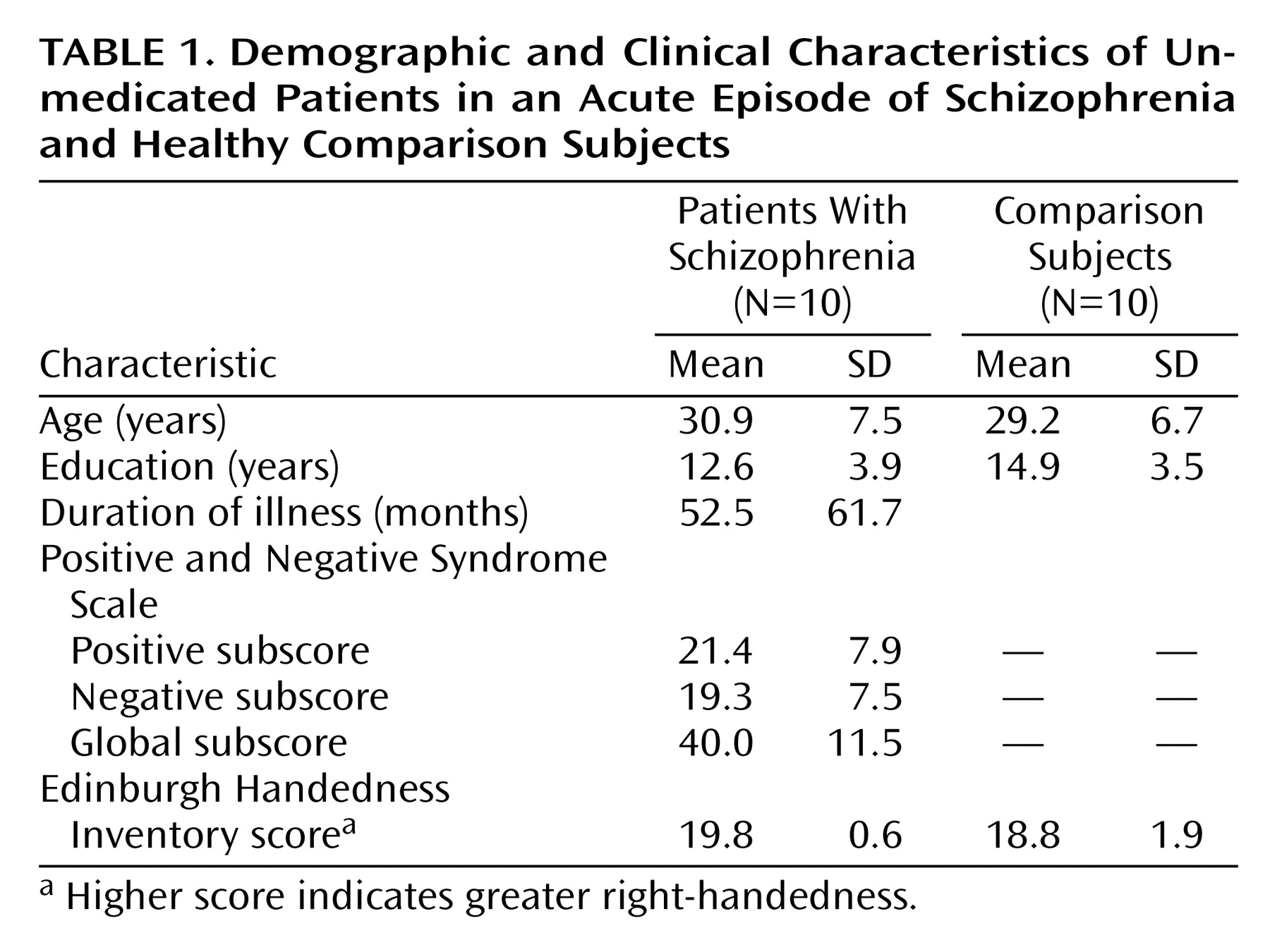
The demographic characteristics of the study group are summarized in
Table 1. There was no significant difference between groups in terms of age, years of education, or task performance. Recognition levels were high for both groups (comparison subjects: mean=91.2% correct [SD=5.5]; patients: mean=77.2% correct [SD=22.6]).
Word Encoding
Regarding frontal lobe activation, comparison subjects showed effects in bilateral ventrolateral (bilateral Brodmann’s area 44, right Brodmann’s area 47) and anterior (bilateral Brodmann’s area 10) regions and in the left anterior cingulate (Brodmann’s area 32). Effects were also seen in the sensorimotor cortex (bilateral Brodmann’s area 6, left Brodmann’s area 4), in the left angular gyrus (Brodmann’s area 39), and in the left lenticular nucleus. In patients, activation was detected in the left precentral gyrus (Brodmann’s area 6) and in the left precuneus (Brodmann’s area 31).
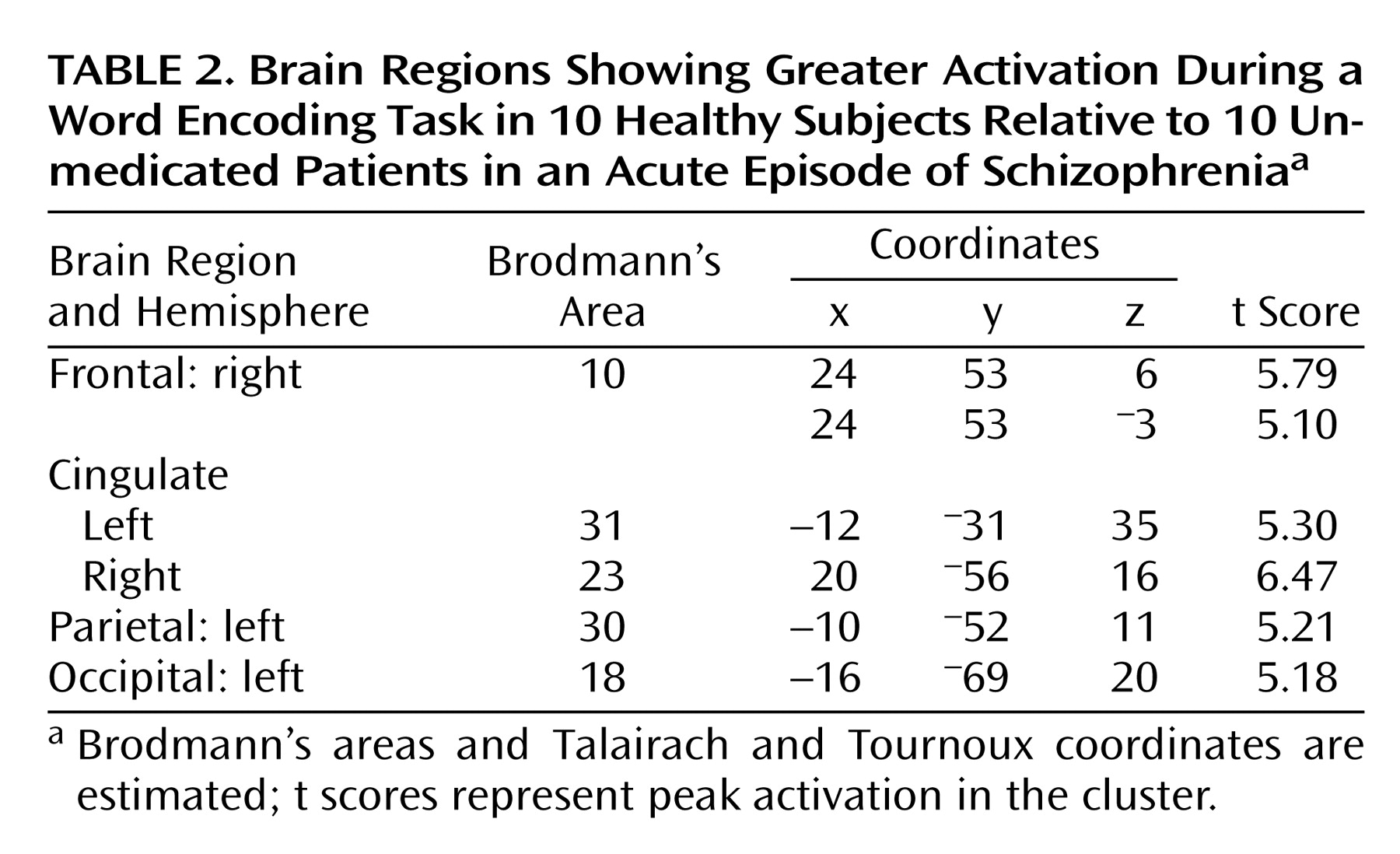
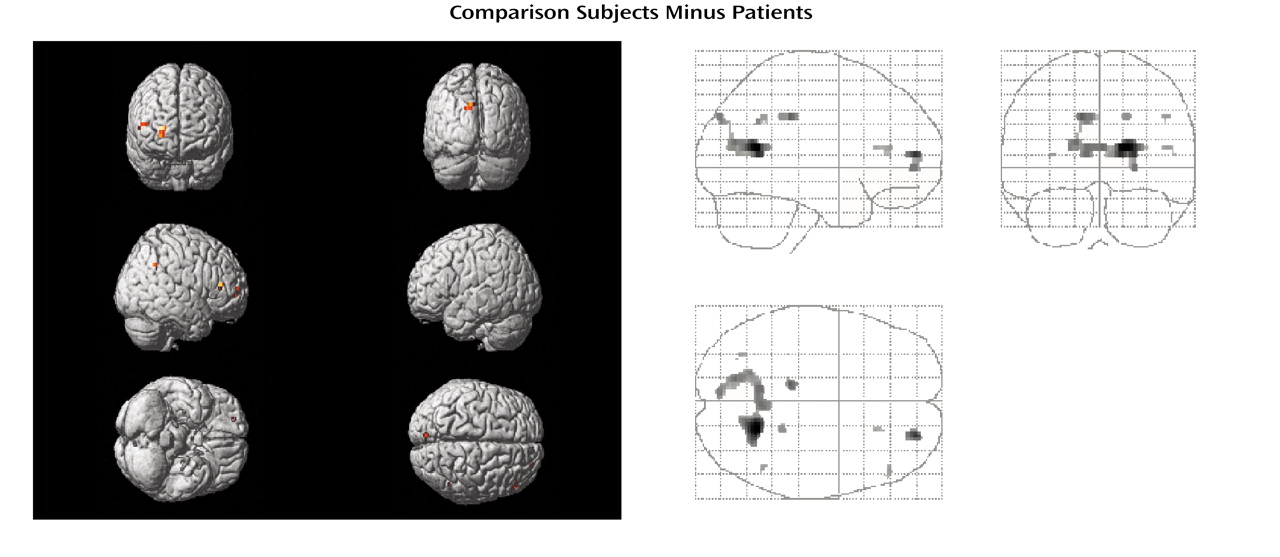
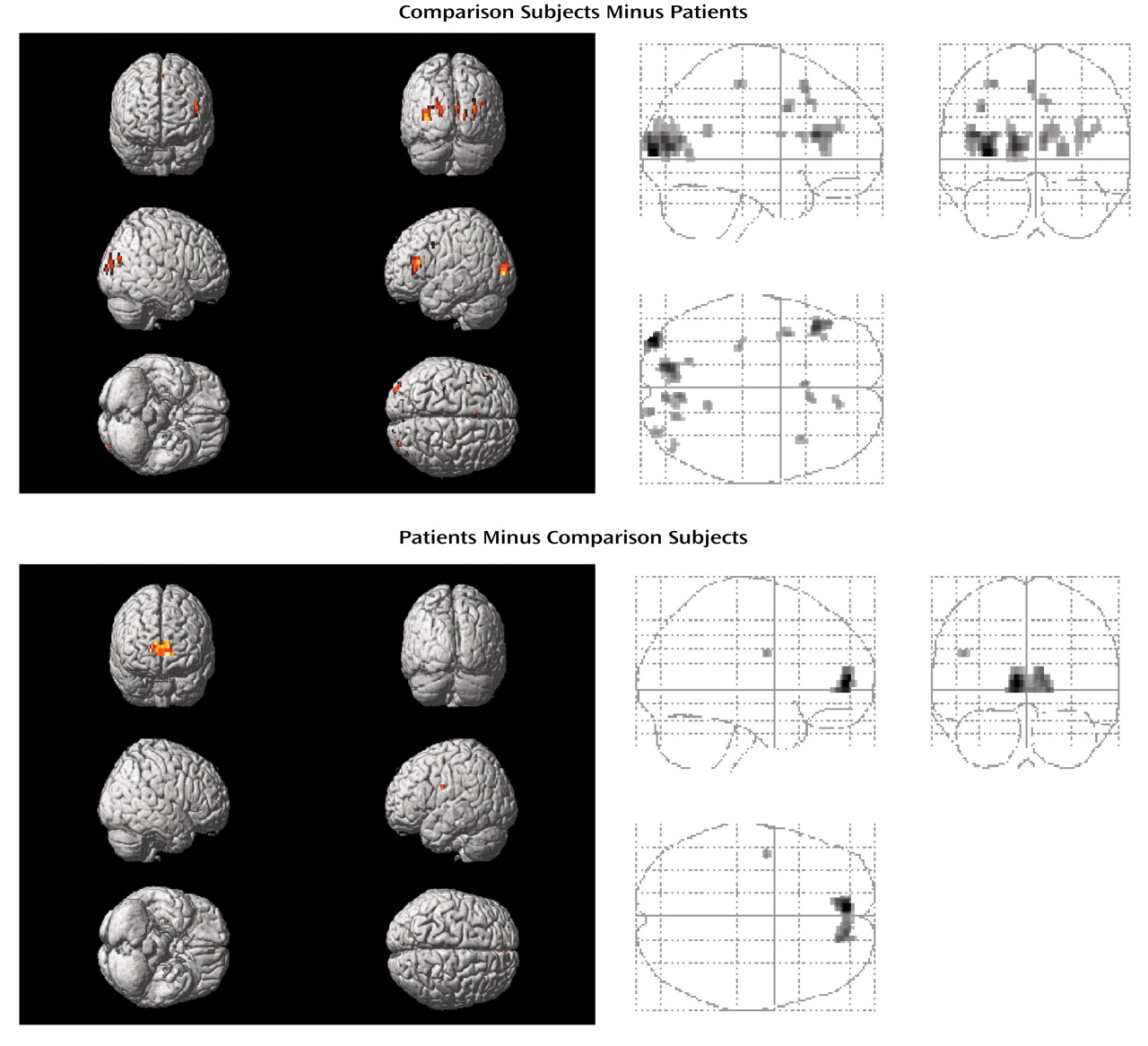
Differences in task-related activation were examined by subtracting the CBF values of patients from those of healthy subjects (
Table 2,
Figure 1). The comparison group had relatively greater activation in the right anterior prefrontal cortex (Brodmann’s area 10), bilateral posterior cingulate (left Brodmann’s area 31, right Brodmann’s area 23), left retrosplenial area (Brodmann’s area 30), and left cuneus (Brodmann’s area 18). The subtraction of activation values of healthy subjects from those of patients yielded no significant differences.
Word Recognition
Word recognition activated a widespread area in both hemispheres. The comparison subjects showed bilateral ventrolateral (bilateral Brodmann’s area 47, right Brodmann’s areas 44 and 45) and left-hemisphere dorsolateral (Brodmann’s area 46) and dorsal (Brodmann’s area 8) prefrontal activations. Further effects were found in left sensorimotor (Brodmann’s areas 4, 6, 2, and 40) regions, bilateral anterior cingulate (Brodmann’s area 32), bilateral lateral temporal cortices (left Brodmann’s area 21, bilateral Brodmann’s area 22), left precuneus (Brodmann’s areas 7 and 19), and bilateral occipital cortices (bilateral Brodmann’s area 18, right Brodmann’s areas 19 and 17). Additional effects were detected in the left thalamus and subthalamic nucleus and in bilateral lenticular nuclei. In patients, activation was seen in bilateral anterior (Brodmann’s area 10) and dorsolateral prefrontal (Brodmann’s area 9) regions, in bilateral precentral gyri (Brodmann’s area 6), and in the left ventrolateral prefrontal cortex (Brodmann’s area 44). In addition, activation was found in the right anterior cingulate (Brodmann’s area 32), the right primary auditory cortex (Brodmann’s area 41), left precuneus (Brodmann’s area 7) and inferior parietal lobule (Brodmann’s area 40), bilateral occipital cortices (bilateral Brodmann’s area 18, left Brodmann’s area 17, right Brodmann’s area 19), in the left thalamus, bilateral subthalamic nuclei, and in the left lenticular nucleus.
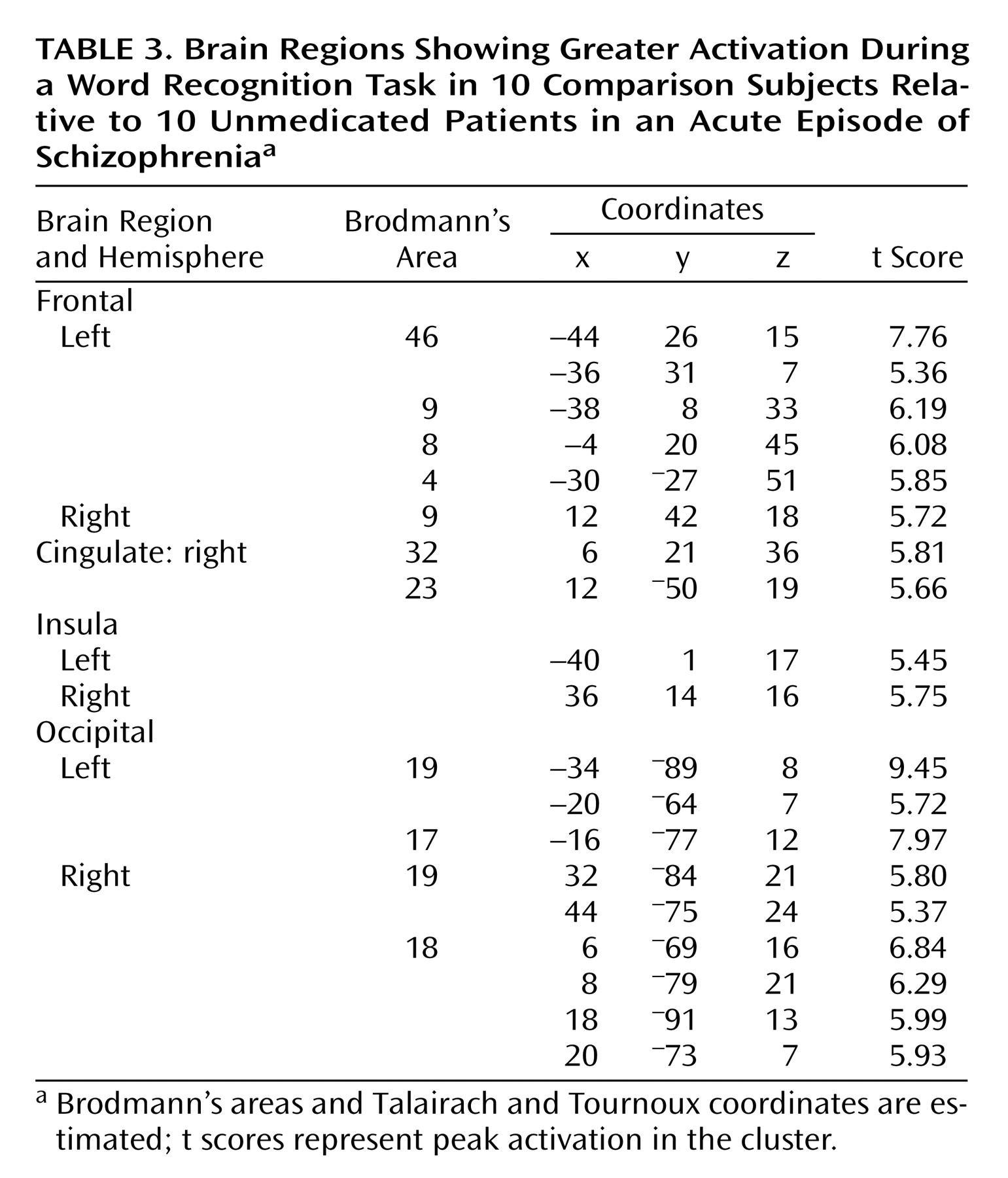
When subtracting the activation values of patients from those of healthy subjects, the comparison group had relatively greater activation in bilateral dorsolateral prefrontal cortices (bilateral Brodmann’s area 9, left Brodmann’s area 46), left dorsal prefrontal (Brodmann’s area 8) and motor (Brodmann’s area 4) regions, right cingulate gyrus (Brodmann’s areas 32 and 23), bilateral insula, and in bilateral occipital cortices (bilateral Brodmann’s area 19, left Brodmann’s area 17, right Brodmann’s area 18) (
Table 3,
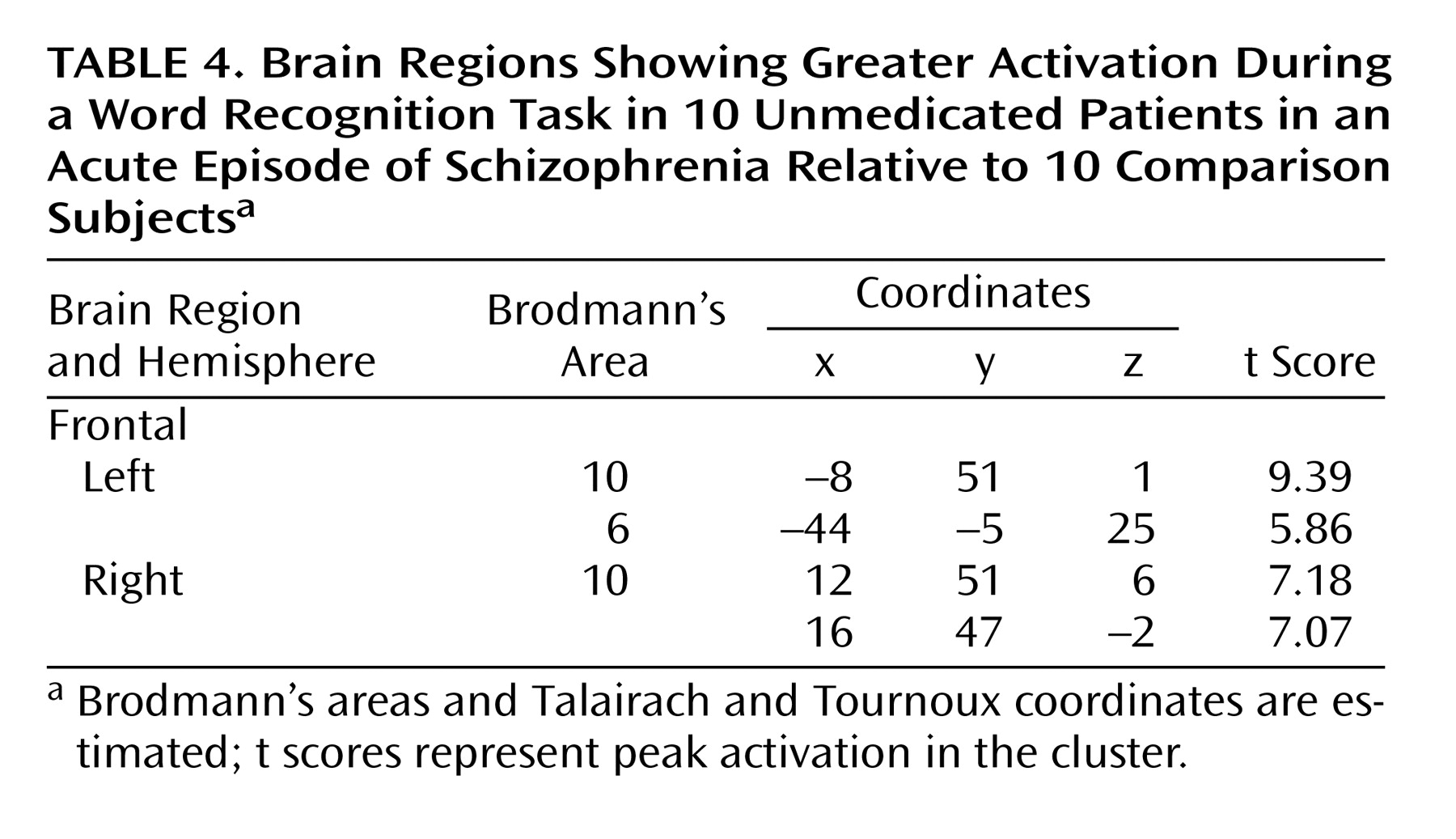
Figure 2). On the other hand, patients with schizophrenia showed a greater activation in bilateral anterior prefrontal cortices (Brodmann’s area 10) and in the left Brodmann’s area 6 relative to comparison subjects (
Table 4,
Figure 2).
Direct comparisons of the same task between patients and healthy subjects revealed the same activation patterns as the task minus rest comparisons.
Discussion
This study examined the neural correlates of episodic encoding and recognition of words in unmedicated, acutely ill individuals with schizophrenia. During encoding, patients with schizophrenia showed a failure to engage frontal, posterior cingulate, and retrosplenial regions. Recognition on the other hand was associated with reduced activation in dorsolateral prefrontal and limbic/paralimbic (cingulate, insula) regions, despite intact recognition performance relative to healthy subjects. In contrast, a higher metabolism in bilateral anterior prefrontal cortices was observed in schizophrenia subjects.
The areas activated by these two tasks in healthy volunteers have been described previously
(21–
33). During recognition, relatively lower activity was seen in the patients’ dorsolateral prefrontal cortex. This region has been associated with verbal working memory in healthy individuals
(34), and correspondingly, impairments of working memory and executive functions in schizophrenia have been associated with decreased blood flow in dorsolateral prefrontal cortex
(35). Our finding of reduced activation in this region in patients might, therefore, be related to impaired working memory in schizophrenia. On the other hand, recognition levels were comparable in both groups. This finding is in agreement with our previous study in clinically stable schizophrenia patients treated with second-generation antipsychotics
(3). We suggest that, despite acute psychosis, our patients’ working memory abilities permitted successful encoding and that reduced dorsolateral prefrontal activation among our patient group may represent executive difficulties.
Our finding of reduced activity in the patients’ anterior prefrontal, posterior cingulate, and retrosplenial cortices during encoding seems surprising at first sight, since these areas have mainly been associated with episodic memory retrieval
(36). However, when interpreting our data, one has to consider that in the current study all material was familiar, since all subjects had already performed the words subtest of Warrington’s Recognition Memory Test before scanning. As the primary scope of this study was to look for differences of functional networks employed to process the tasks in question and not to look for cognitive differences between patients with schizophrenia and healthy comparison subjects, this procedure was chosen in order to guarantee task understanding. Obviously, we have to assume retrieval processes, which might have occurred while the participants performed the encoding task in the scanner. Consequently, our finding of relatively greater right anterior prefrontal activation during the encoding task in the comparison group may represent retrieval processes. Accordingly, our finding of enhanced activity in bilateral anterior prefrontal cortices among the schizophrenia group during the recognition task may refer to retrieval success
(37). We speculate that the relatively small time period between the encoding and recognition tasks during scanning enabled our patients to maintain the encoded information in working memory and to engage retrieval processes. Since we did not observe such findings in remitted patients with schizophrenia
(3), acute psychosis may have prevented practice effects in the present patient group. This hypothesis agrees with our finding of greater posterior midline activation in the comparison group during the encoding task, which has also consistently been associated with successful episodic memory retrieval
(36).
Reduced activation was also found in the anterior cingulate of patients during performance of the recognition task. Disturbances in this brain region have been reported in patients with schizophrenia during several cognitive tasks
(8,
38–41). Next to its participation in an “attentional system,” this structure has been proposed to contribute to the recognition of newly learned material
(42). Furthermore, the anterior cingulate has been said to play a prominent role in the executive control of cognition
(43). The lack of anterior cingulate activation in our patient group may therefore reflect an attentional deficit on one hand and executive difficulties on the other.
Patients also showed reduced bilateral insula activation during the recognition task. This structure has been associated with semantic encoding, short-term storage of auditory-verbal material, and internal phonological processing of written words in healthy individuals
(44–
46). Deficits in insular activity of schizophrenia patients have also been previously reported by other groups
(8,
47). However, our finding should be viewed with caution given the fact that comparison subjects did not show insular activation during the within-group analysis. Still, our results underscore the importance of this structure for the pathophysiology of schizophrenia.
The current study has several limitations. First, we have studied a small group of subjects, and all subjects were male. Gender differences in the functional organization of the brain for working memory have been shown in healthy individuals
(48), while in schizophrenia, further studies are needed to elucidate the impact of gender on memory performance and brain activation. Second, the current study included patients with schizophrenia who were at different stages of the illness (first-episode and multiple-episode patients), and only the first-episode patients were neuroleptic naive. We cannot exclude an influence of previous antipsychotic exposure on the cerebral activation pattern in the multiple-episode patient group, even though they were free of antipsychotics at the time of study. Third, all subjects had already carried out the task before scanning. Activation patterns observed in our group may therefore be different from those that would have emerged without this test run. On the other hand, use of a prescan test may eliminate artificial findings resulting from mere differences in being able to follow test procedures between study participants.
Last, we chose to contrast encoding and recognition with a resting baseline reference condition rather than performing serial subtractions of increasingly complex reference tasks
(49) and were therefore not able to control for nonmemory functions. This procedure might be responsible for the lack of visual cortex activation for the task versus rest comparisons for both patients and comparison subjects during the encoding task, since subjects may have used visual imagery to rehearse the presented words, which would have canceled out visual activation during the presentation of the words. However, the use of active reference conditions has the potential limitation of confounding the interpretation of activation effects if cognitive components interact between one or more reference tasks
(50). Nevertheless, including a motor baseline to our resting baseline task in order to control for motor activation during the acquisition and recognition conditions would have strengthened the interpretation of our findings.
Our findings are of considerable clinical relevance insofar as verbal memory has been found to be related to community outcome, social problem solving, and the acquisition of social skills in patients with schizophrenia
(51). One challenge for future imaging studies will be to find out whether and to what extent cognitive dysfunction in schizophrenia is caused, improved, or worsened by medication. This calls for longitudinal studies that investigate patients before and after the implementation of treatment.