We examined hemodynamic effects associated with brief episodes of visually driven and fast-paced digit sequence learning in patients with autism and healthy comparison subjects. Consistent with previous studies
(53–
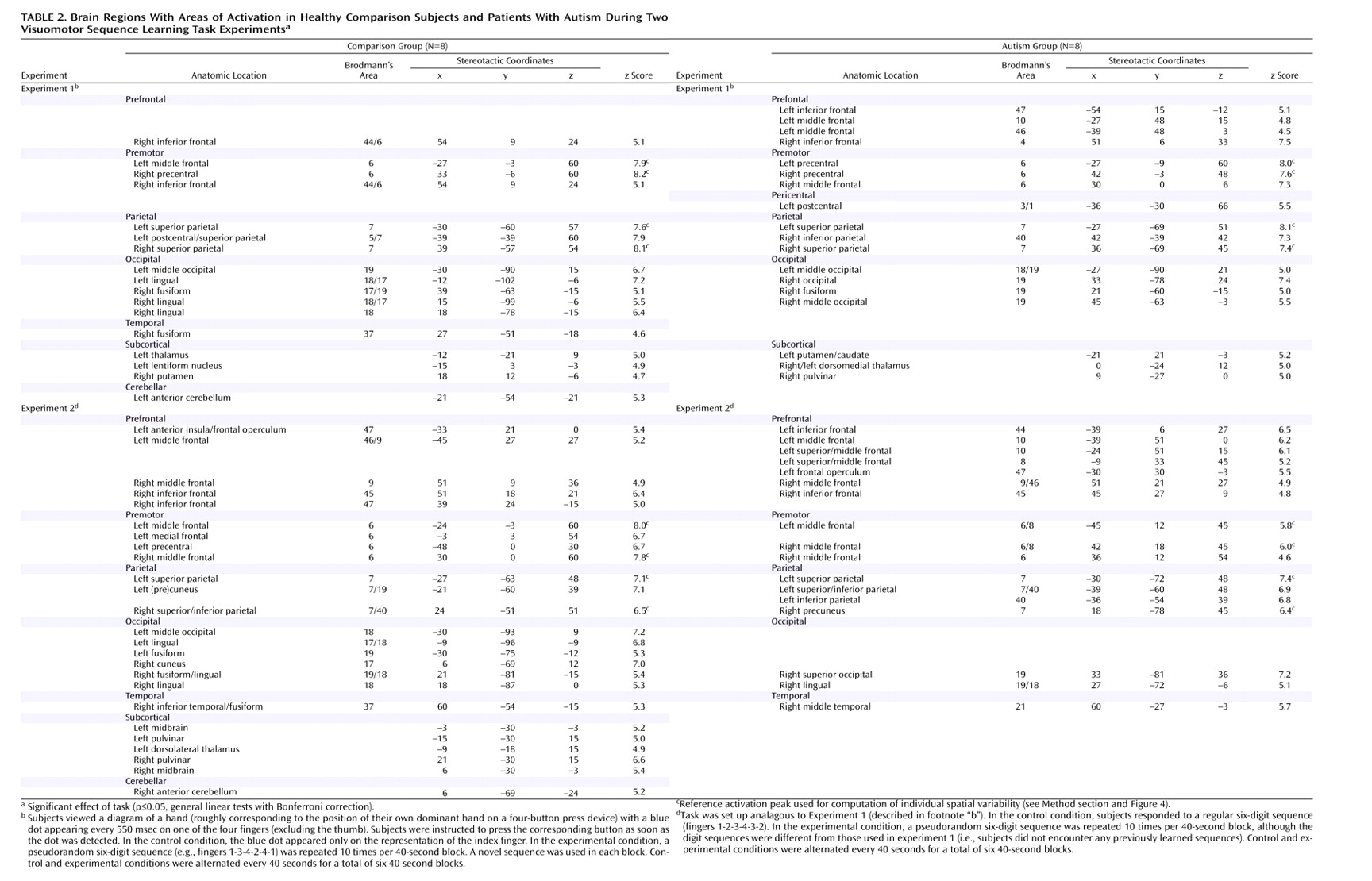
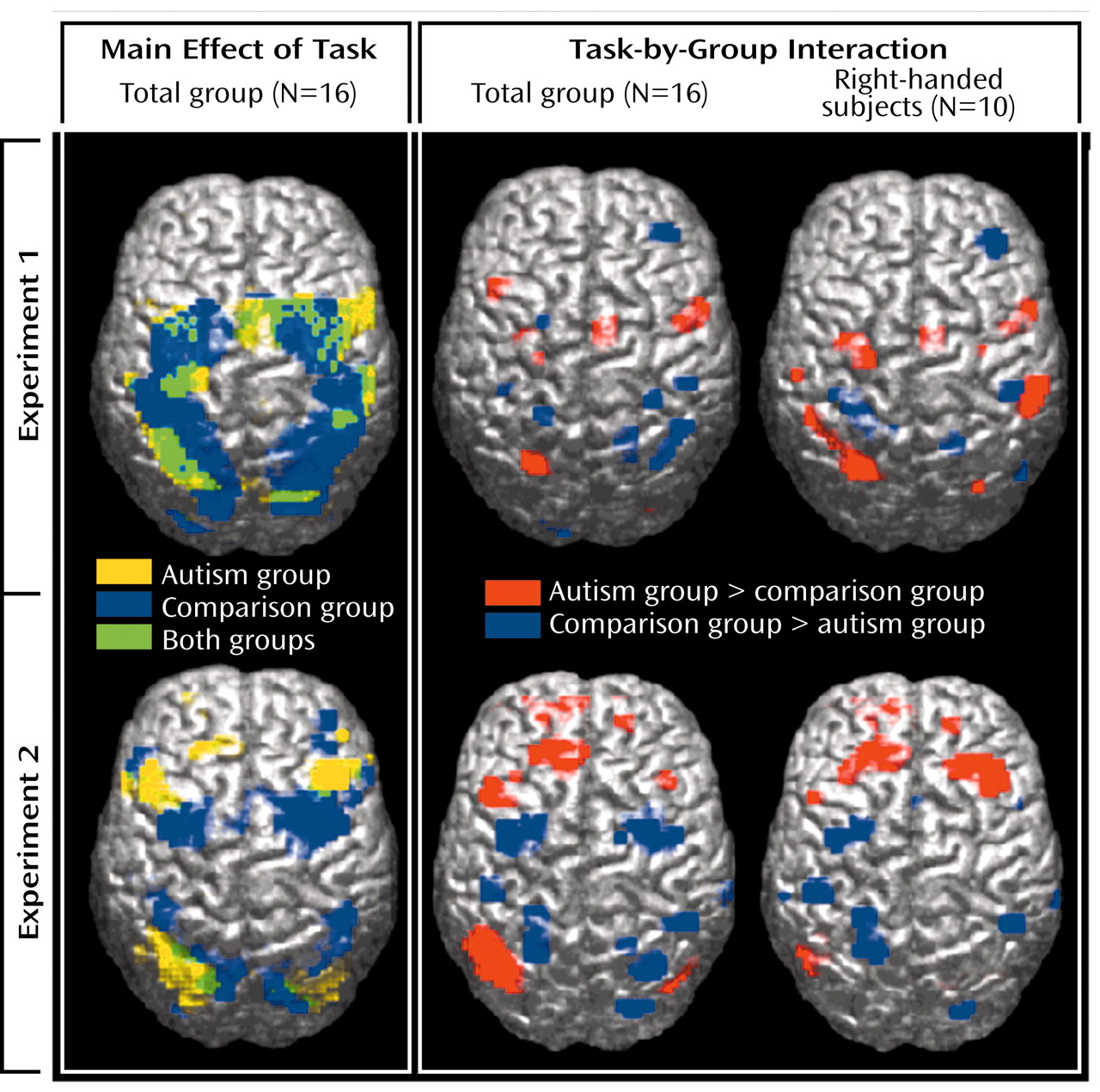
55), we identified extensive involvement of the premotor and parietal cortex. Even though activation patterns overlapped across groups, in particular in experiment 1 (
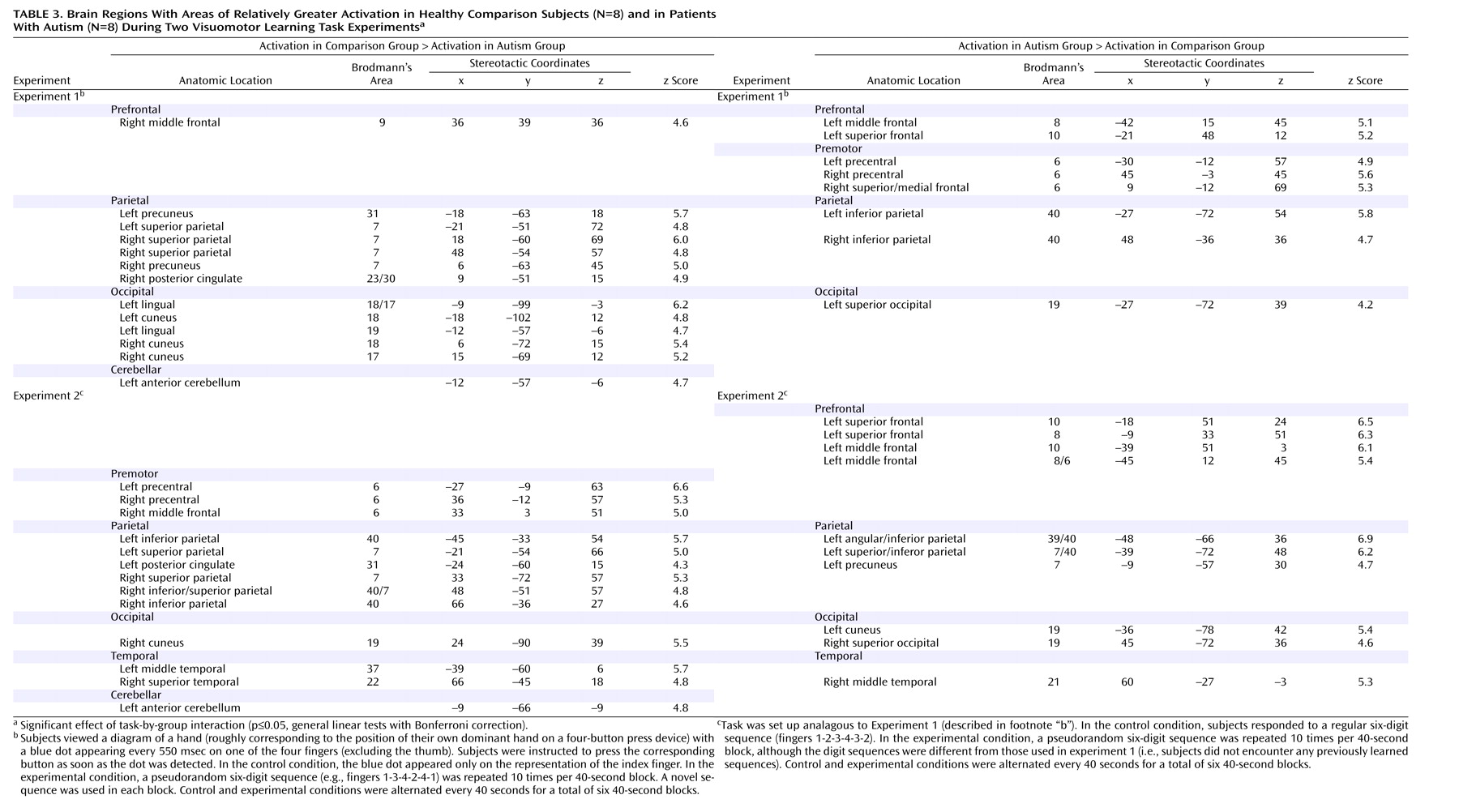
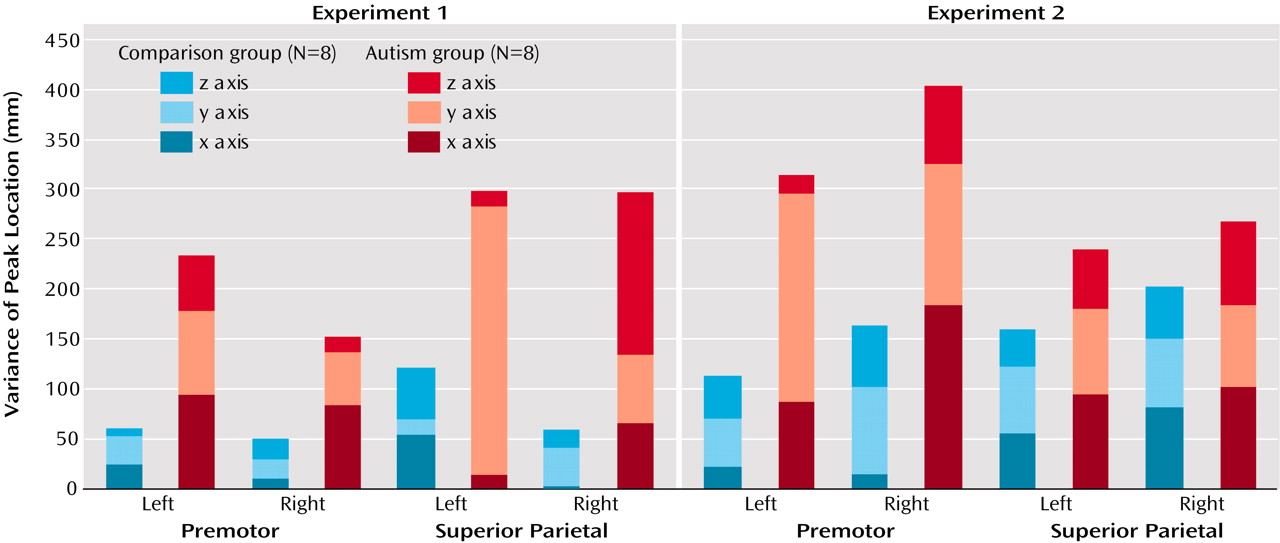
Figure 1, upper left panel), significant effects of task-by-group interaction were found, mostly in the frontal and parietal lobes. With the single exception of a site in the right middle frontal gyrus (area 9), which showed greater activation in the comparison subjects in experiment 1, autistic patients showed overall stronger prefrontal involvement during visuomotor learning. This group difference was especially pronounced in experiment 2, which was less sensitive to effects of motor execution. In premotor cortex (area 6), we found similar effects in experiment 1 but inverse effects in experiment 2 (stronger activation in the comparison group).
These apparently inconsistent findings are most likely explained by more distributed and scattered cortical recruitment during simple finger movement, as previously reported
(29). In experiment 1, the task was motorically more complex than the control condition. Abnormal scatter of motor activation beyond the primary motor cortex would thus explain the observed greater premotor effects in the autism group. Frontal group differences in experiment 2 can be explained analogously, again assuming scatter of functional maps in autism. The bilateral premotor cortex is a consistent site of activity related to motor learning in normal adults
(53,
55–58). In autistic patients, scattering of activity related to motor execution into the premotor cortex
(29) would be subtracted out in experiment 2 (because motor execution was fully balanced across the task and control conditions). Scatter of activation more specifically related to visuomotor coordination and learning, however, would be associated with reduced activation in the premotor cortex itself but abnormal recruitment of neighboring prefrontal regions anterior to the premotor cortex. This pattern of group effects was indeed observed, consistent with the general hypothesis of individually variable scatter of functional maps discussed later in this section.
Bilateral activation in the parietal cortex was found in both groups, as expected
(53,
59–61) (
Figure 1, upper left panel). Involvement of the superior parietal area (area 7) in normal adults can be attributed to visuospatial attention during early stages of digit sequence learning. Our group
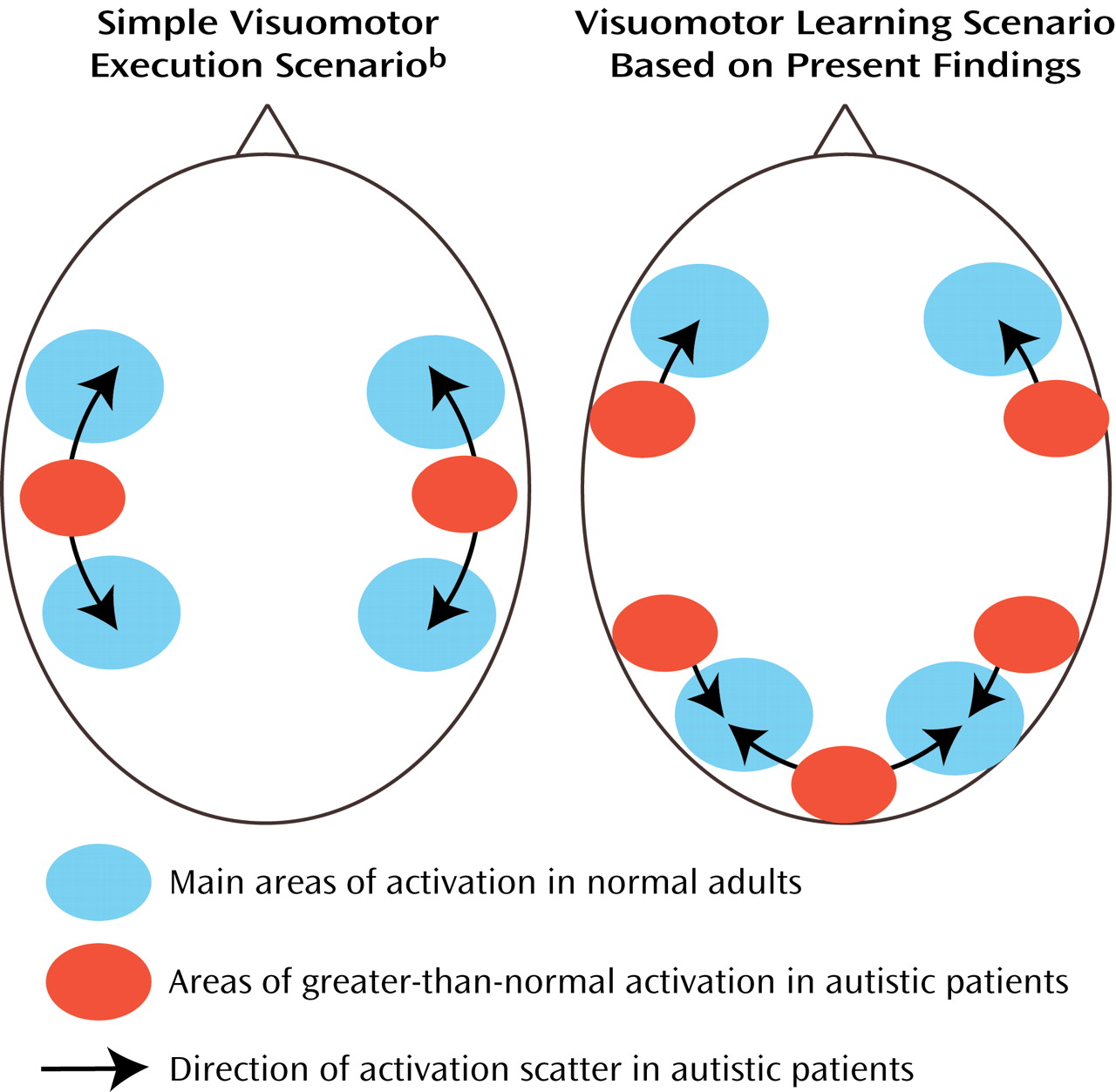
(58) recently reported bilaterally stronger activation in area 7 for early compared to later stages of digit sequence learning, associated with upregulation of visual processing in the striate and extrastriate cortices, which is also characteristic of early stages of visuomotor learning. The finding of more posterior and inferior activation in the autism group is again consistent with a general hypothesis of scattered maps. Activation scatter would result in reduced activation in the normal superior parietal and occipital foci but abnormal recruitment of cortical tissue
between these regions (
Figure 6).
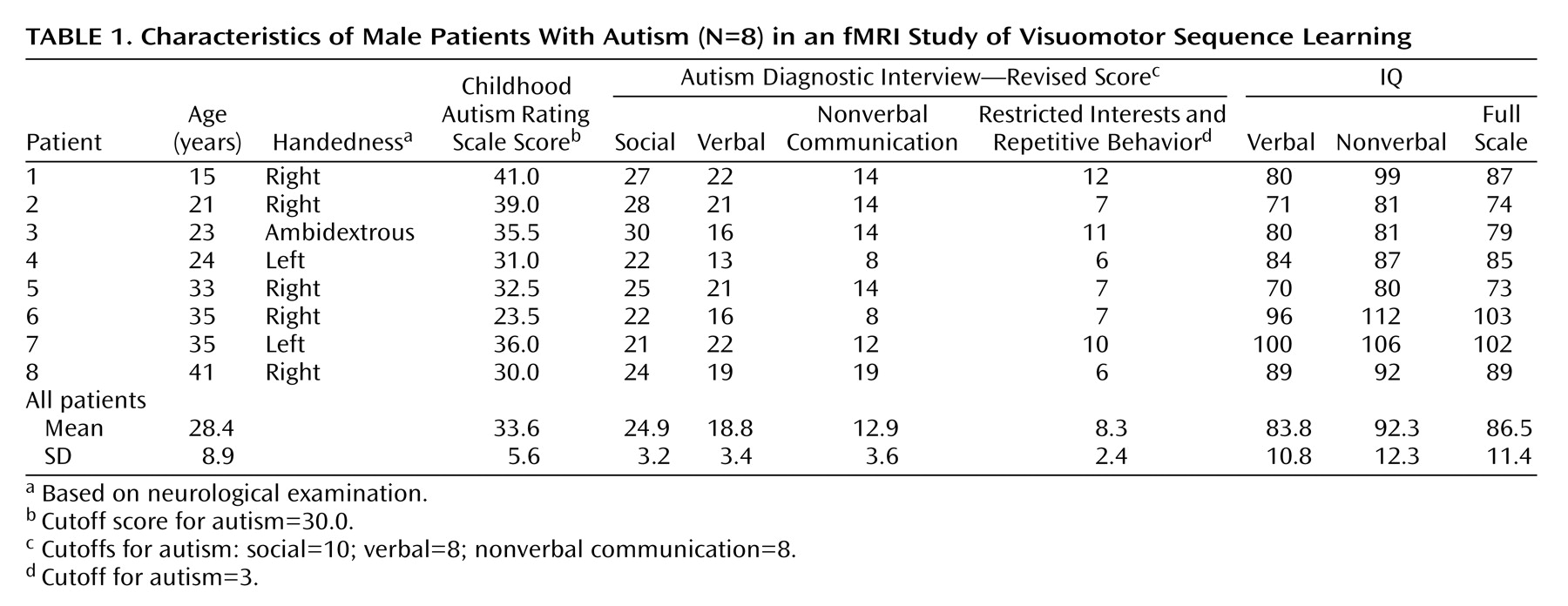
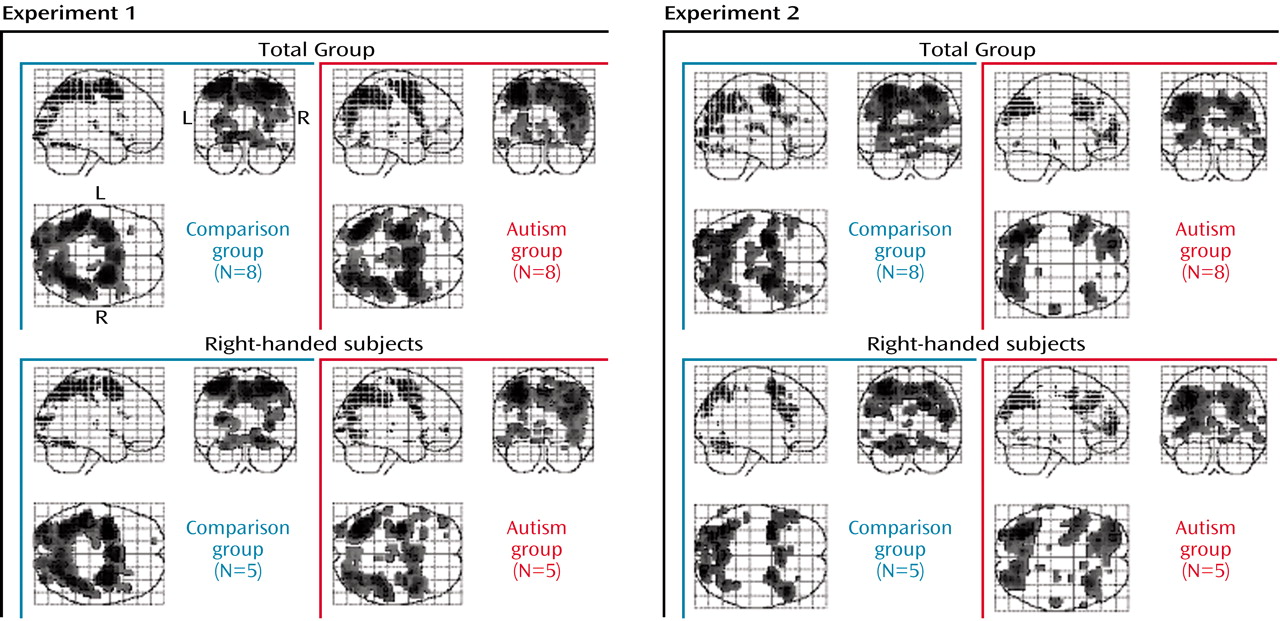
Each group included three non-right-handed subjects. In additional analyses of data for right-handed subgroups (N=5 in each group), the main effects of task were very similar to those for the full groups, albeit slightly weaker (due to reduced statistical power) (
Figure 2). Specifically, effects showed no laterality differences, suggesting that our main findings were not affected by handedness or side of movement. Finally, to rule out potential confounds of task performance, we analyzed data for subgroups that were grossly matched for response times and errors (N=6 per group). Despite some minor differences, the main findings for the frontal and parietal lobes described earlier were consistent (
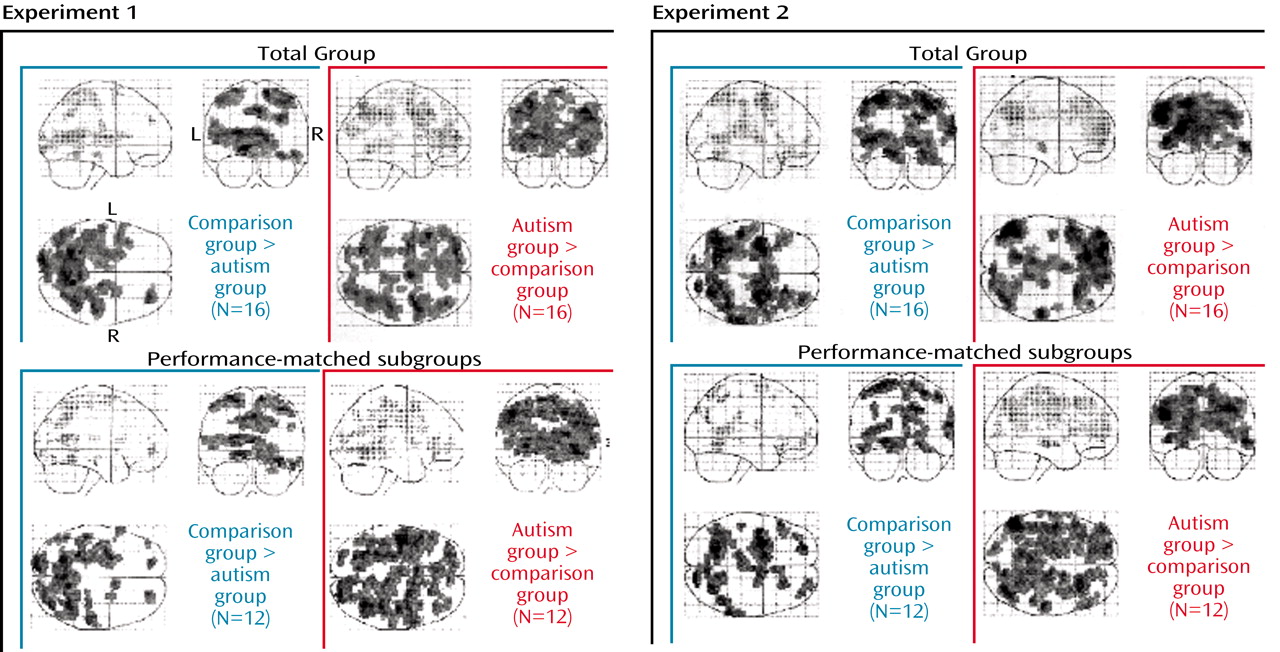
Figure 3). It should be noted that similar levels of performance in these subgroups cannot absolutely guarantee absence of confounds regarding, for example, attention, general arousal, or differences in cognitive strategies.
A Developmental Account
In each of the few published functional mapping studies of autism and Asperger’s disorder
(20–
23,
25,
29,
65,
73), significant differences from normal hemodynamic responses to a variety of tasks have been observed. Such abnormalities support the expectation that autistic pathogenesis is associated with neurofunctional disturbances. However, ascertaining neurological abnormality in a disorder that by consensus is neurally based is not truly enlightening. Some studies have demonstrated reduced or absent activation in regions that are functionally well characterized in normal brains, such as the fusiform gyrus for face perception
(24,
25) or the amygdala for emotional processing
(21). Even though such findings may be linked to respective deficits in autism, the view that recruitment of differently localized cortical tissue would necessarily reflect functional impairment remains an assumption. Given our insufficient understanding of neurofunctional organization in autism and the known multipotential qualities of developing cortical tissue (see later discussion), this assumption is speculative. In addition, the vast array of sensorimotor and cognitive disturbances observed in autism makes it unlikely that this disorder can be neurofunctionally described in terms of a localized or “modular” deficit.
In view of the pervasive developmental nature of the disorder, the question of abnormal neurofunctional maps observed in autistic adults must be approached developmentally: How do functional maps differentiate in normal development, and how do developmental disturbances in autism interfere with this normal differentiation? Since fMRI studies of autistic patients at the time of initial pathogenesis (prenatal or early postnatal) are impractical, developmental considerations can apply only post hoc. Autism is a behaviorally defined psychiatric disorder, and diagnostic criteria may not correspond to a unique developmental pathology. On the contrary, the diversity of findings in structural
(5,
74) and functional imaging
(19) and in genetic linkage studies
(75,
76) suggests that multiple etiological pathways result in phenotypes captured under the diagnostic umbrella term of autism. In this context, it becomes crucial for neuroimaging studies to examine data on an individual level. To our knowledge, only three studies have attempted this approach.
A study of face perception
(25) showed that absence of normal activation (in the fusiform gyrus) on groupwise analyses does not imply absence of activation in individual autistic patients. Instead, the majority of patients in this study showed activation in individually varying loci around the fusiform gyrus. Our previous study of index finger movement
(29) demonstrated abnormal individual variability of activations in autism for simple motor control, with individual maps showing scattered activation beyond the perirolandic cortex. Very similar effects were also observed in the cerebellum in a separate study of simple thumb movement
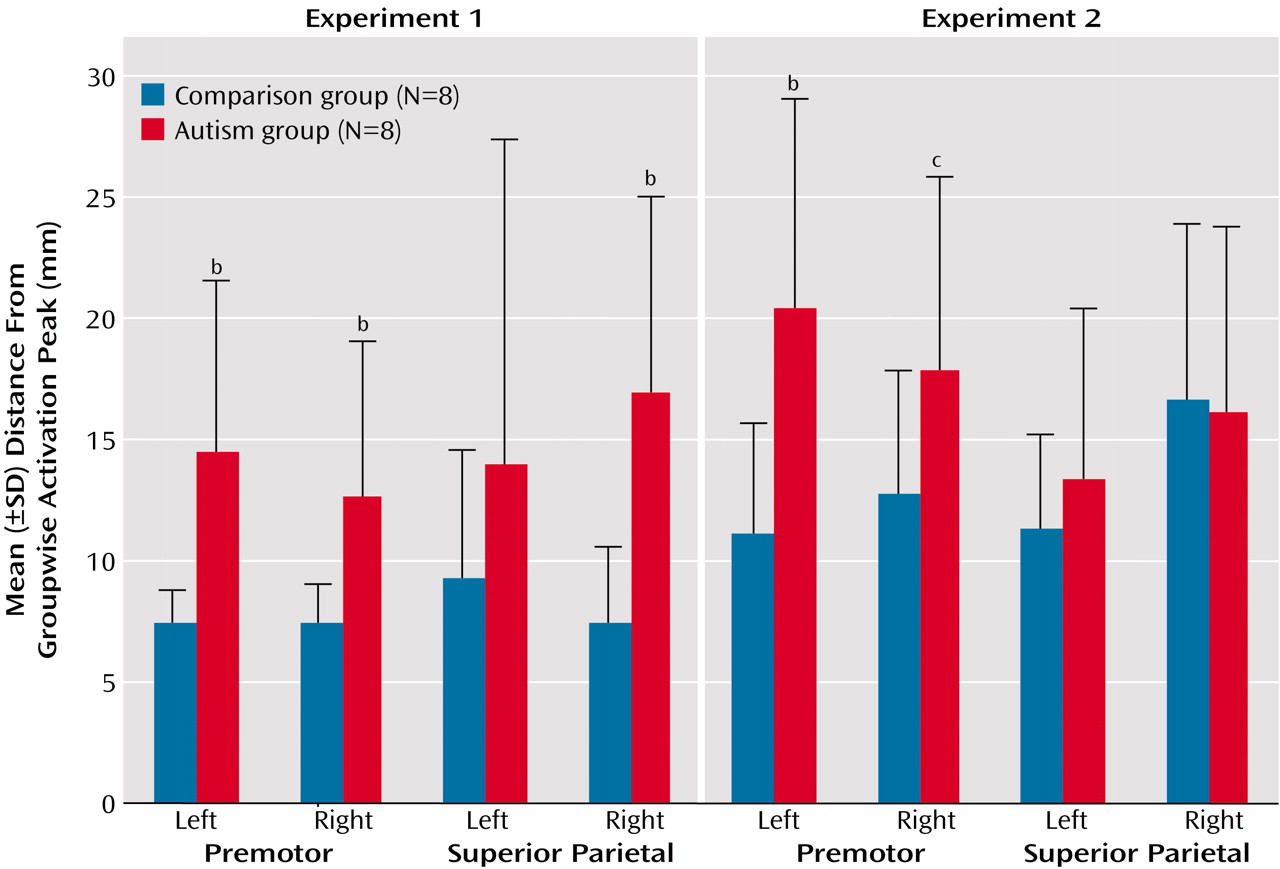
(28). Among the three previous studies examining fMRI findings on a patient-by-patient basis, there was thus complete convergence indicating individually variable scatter of activation beyond the foci seen in normal subjects. In the present study, we again found overall significantly greater spatial variability of activation foci in the autism group, compared to the healthy subjects (
Figure 5 and
Figure 6), which supports our hypothesis that such variability may be a general characteristic of neurofunctional organization in autism and may directly reflect developmental disturbances.
Despite important genetic influences on brain morphology
(77) and cognitive abilities
(78), normal neocortical differentiation into functionally specialized regions is not strictly predetermined genetically
(79,
80). Crossmodal plasticity of developing cortical tissue has been demonstrated in animal models
(81,
82) and in human imaging studies
(83,
84). Animal studies have further established that thalamocortical afferents play an important role in such differentiation
(81), as well as in lesion-induced cortical plasticity
(85). Intact thalamocortical afferents are thus a prerequisite for the normal development of cerebral cortical functional maps. There is some, as yet limited, evidence for thalamic abnormalities in autism
(15,
26,
86–89). It is known that the thalamus participates in extensive cerebello-thalamo-cortical pathways reaching almost all cerebral cortical regions, including association cortex
(90). Among the many sites of neurological abnormality proposed in autism research, the cerebellum has been identified through convergent evidence, including findings of postmortem
(17), volumetric
(10,
91–93), neurochemical
(15,
16,
94), behavioral
(95), and activation studies
(26,
27,
65). Postmortem histology suggests that the most prominent cerebellar abnormality is a reduction in Purkinje cell numbers
(17,
96). Fatemi and colleagues
(94) observed reduced levels of reelin and Bcl-2 protein in autistic postmortem cerebella. These proteins play important roles in migration and cortical lamination (reelin) and apoptosis (Bcl-2). The postmortem findings from adult brains do not establish whether similar regulatory protein reduction could explain developmental abnormalities in the autistic cerebellum. However, an absence of “empty baskets” suggests that Purkinje cell reduction in adults reflects maldevelopment rather than post hoc loss
(17,
97). Growth interaction between basket cells and Purkinje cells occurs before the differentiation of Purkinje cells is completed (around postnatal day 8 in the rat
[98], roughly corresponding to the end of the second trimester in human gestation
[99]). Inhibitory synapses of Purkinje cells in deep cerebellar nuclei probably form around the beginning of the third trimester in humans
(99,
100). In the thalamus, afferents from deep cerebellar nuclei are present at birth in the rat, i.e., preceding the events described earlier
(101). Early Purkinje cell loss in autism is thus likely to affect developing cerebello-thalamo-cortical connectivity and cerebral cortical functional differentiation, which may explain abnormally scattered fMRI activation patterns, as observed here.
Representational scatter in autism (as depicted in
Figure 6) may imply phenomena analogous to those of “crowding” suggested for lesion-induced interhemispheric reorganization
(102,
103). Currently limited fMRI evidence suggests that ontogenetically earlier functions (e.g., simple motor control, visual perception) invade brain areas normally involved in more complex multimodal processing. The opposite is not observed. For instance, we did not see greater-than-normal activity in the primary motor cortex associated with visuomotor coordination. Instead, scatter was directed toward the prefrontal cortices, i.e., the relatively late-maturing cortices that normally assume higher “executive” functions. Our hypothesis implies that due to impaired cerebral cortical differentiation (caused at least partly by disturbances in thalamocortical afferents), early-developing sensorimotor functions require a larger cortical processing territory. Such territory will subsequently be less available to later-developing complex multimodal processing (e.g., language, executive functions). This hypothesis of
hierarchical crowding thus implies that more elementary functions impinge on more complex ones in the developing autistic brain.
This scenario is not a fully comprehensive model of autistic neuropathology. Relatively elementary functions (such as motor dexterity, auditory perception, spatial attention) are often impaired in autism, whereas some high-functioning patients, including those with Asperger’s disorder, perform well in higher cognitive domains. The hypothesis of hierarchical crowding does not address potential effects of compensatory neuroplasticity, known from the literature on patients with early brain damage
(104,
105). Furthermore, given the plethora of pathological findings in autism, additional etiological mechanisms besides early Purkinje cell reduction and hypothesized cerebello-thalamo-cortical disturbances—such as diffuse growth disturbances
(91,
106)—are likely to play important roles.
It should be noted that the proposed model has been prompted by imaging findings in rather small groups of high-functioning autistic patients, and replication will be required. Nonetheless, our model generates a number of hypotheses that may inform future fMRI work. To examine these hypotheses, it will be important to go beyond the customary procedure of groupwise analyses and examine the autistic brain on an individual level. First, our model implies predictions for fMRI studies on elementary and complex cognitive functions in various domains and modalities. Second, the model would predict functional impairment of the thalami beyond the currently limited evidence. Third, it is predicted that axonal pathways connecting cerebellar deep nuclei, the thalamus, and the cerebral cortex are affected.