Autism is a severe pervasive developmental disorder characterized by impairments in reciprocal social relationships, verbal and nonverbal communication, and the ability to play and to develop interests outside of stereotypic preoccupations
(1). Autism is also characterized by disturbances in the perception and modulation of sensory information, especially in the auditory domain. For example, young autistic children are often initially misdiagnosed as deaf
(2). Two independent studies
(3,
4) have reported bilateral hypoperfusion at rest in the temporal lobes of children with primary autism. In our study
(3), abnormalities were centered on the multimodal superior temporal sulcus and in the auditory cortex of the superior temporal gyrus
(3). However, up to now only two auditory functional brain imaging studies have been performed in autistic patients
(5,
6). Thus, in the present positron emission tomography (PET) activation study, we further test auditory cortical processing in autism by using a passive listening task of complex speech-like auditory stimuli. We have previously shown in healthy subjects that these stimuli activate large areas of the superior temporal cortices
(7), which are selectively involved in the initial “acoustic” stage of speech perception
(8). These stimuli are never recognized as speech and are therefore unlikely to be explicitly processed by semantic language systems. Therefore, with this paradigm, the putative cortical activation differences between autistic patients and healthy subjects may reflect basic anomalies of cortical prelinguistic auditory processing rather than consequences of abnormal language development.
Method
We studied five adults (four men and one woman) with a primary autistic disorder (mean age=19.1 years [SD=4.5]; mean IQ=64 [SD=5]). Autism was diagnosed according to DSM-IV criteria. Autism was confirmed in all patients with the Autism Diagnostic Interview—Revised
(9) (social interaction score: mean=25, SD=3.8; nonverbal communication score: mean=10.4, SD=2.5; stereotypy score: mean=6.2, SD=2.3; age at onset: mean=3.6 years, SD=1.3). Four patients had typical autistic speech abnormalities (verbal perseveration, stereotypy, echolalia, abnormal prosody, neologism), and one patient had no communicative speech. We excluded from this study patients with infectious, metabolic, neurological, or genetic diseases and those with abnormal EEG or MRI results. All patients were medication free for at least 1 month before the PET scan.
Eight healthy male university students were volunteers for this study (mean age=21.9 years, SD=3.3). They had no history of neurological or psychiatric disorders.
All autistic and comparison subjects had normal auditory functioning as assessed by an audiogram. An ethics committee approved this study. Written informed consent from the patients’ parents and from the healthy subjects was obtained after the procedure had been fully explained.
Relative regional cerebral blood flow (rCBF) was determined from the distribution of radioactivity measured with PET (ECAT-EXACT-HR+) after bolus intravenous injection of H
215O
(10). The experimental protocol included three rCBF measurements carried out in a single session, performed at 10-minute intervals. The first measurement occurred during the rest condition, and the second and the third occurred during passive listening to complex speech-like synthetic sounds. During each auditory stimulation, started 25 seconds before image acquisition, stimuli were delivered binaurally at a 68-dB sound pressure level and a 1-second inter-onset interval.
We used a subset of the synthetic speech-like auditory stimuli previously published elsewhere
(7). Briefly, these stimuli contain spectral maxima (like speech formants) changing in time. They consist of complex sounds with a central 200-msec steady-state period surrounded by initial and final changes in frequency of the spectral maxima. Their acoustic structure was very similar to consonant-vowel-consonant, but as stated, normal volunteers never recognized them as speech sounds.
The rCBF images were analyzed with statistical parametric mapping software (SPM 96) used for image realignment, transformation into standard stereotactic anatomical space, smoothing (15 mm), and statistical analysis
(11). State-dependent differences in global flow were covaried out by using proportional scaling. Comparisons across conditions were made with the t statistic subsequently transformed into the normally distributed z statistic by using a multistudy design. The resulting z maps were thresholded at p<0.001 corrected at p<0.05 for multiple comparisons.
Two statistical analyses of activation were performed: a within-group comparison of activation for listening to complex sounds versus the rest condition and a between-group comparison of activation.
Results
Passive listening to speech-like sounds versus the rest condition was assessed in each group independently. In the healthy comparison subjects, there was bilateral superior temporal cortex activation with a left-biased asymmetry (z>3.09, df=22, p<0.001). Five peaks of activation were detected along the left superior temporal cortex, whereas only two peaks were detected in the right superior temporal gyrus (Brodmann’s area 22). The volume of the activation was larger in the left hemisphere (32 cm
3) than in the right (20 cm
3). No peak of activation was detected outside the temporal lobes. This pattern of activation has been previously reported
(7).
There was also bilateral superior temporal cortex activation in the autistic patients, but with a reverse right-biased asymmetry (z>3.09, df=22, p<0.001). Five peaks were detected in the right superior temporal cortex and three peaks in the left superior temporal gyrus. The volume of activation was larger in the right hemisphere (55 cm3) than in the left (19 cm3). In addition, six foci of activation were detected in the right frontal lobe and two in the left precentral gyrus.
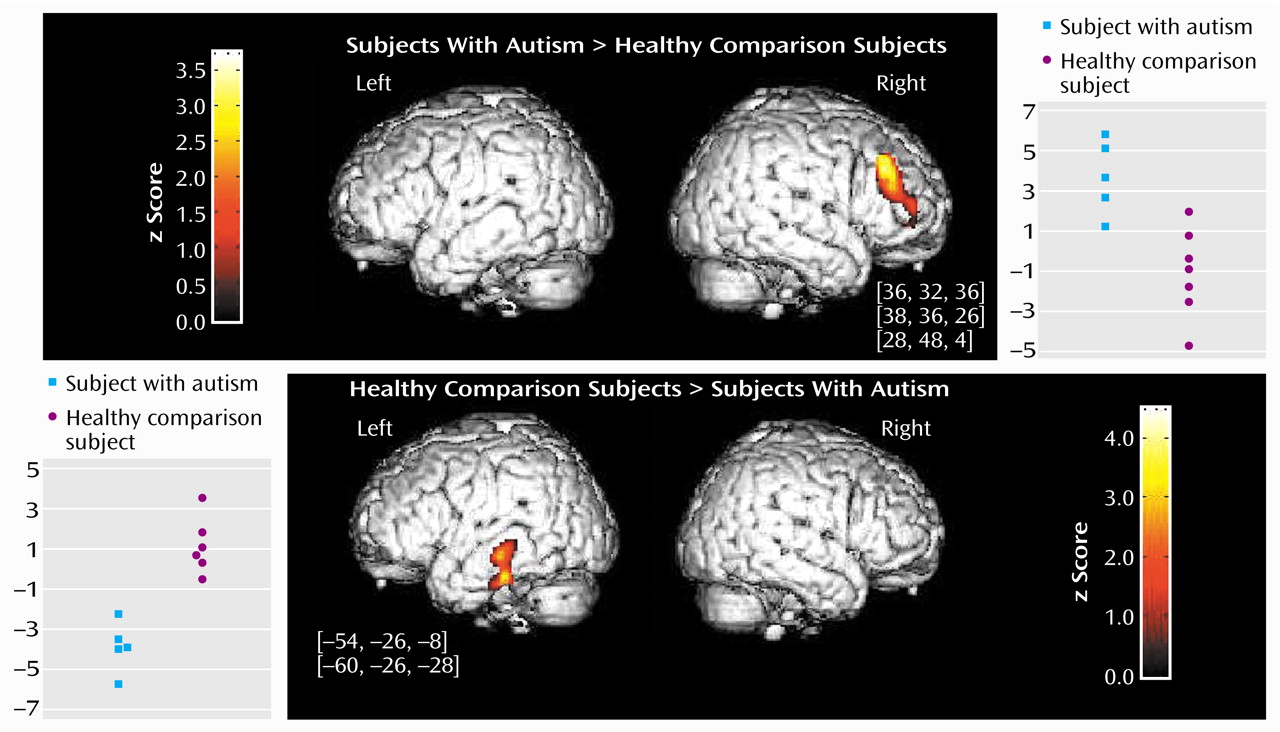
The direct comparison showed a statistically significant difference between the autistic and the healthy comparison subjects in two brain areas (z>3.09, df=22, p<0.001). The right middle frontal gyrus (Brodmann’s areas 9, 9/46, and 10) exhibited significantly greater activation in the autistic patients than in the comparison subjects. In addition, the posterior part of the left middle and inferior temporal gyrus (Brodmann’s area 21) exhibited significantly less activation in the autistic patients than in the comparison subjects (
Figure 1).
Discussion
In this study, we investigated auditory cortical processing in patients with autism by using stimuli designed to investigate early cortical stages of auditory speech processing. In healthy subjects, these stimuli induce bilateral activation of secondary auditory areas located in the lateral belt of the auditory cortex
(7), which are selectively involved in the initial acoustic steps of language processing
(8). In the present study, we observed an abnormal pattern of activation in the autistic patients. At the level of the auditory temporal cortex, our data suggest a reverse hemispheric dominance, since the volume of activation was larger in the right auditory cortex of autistic subjects, whereas the reverse pattern was found in the comparison subjects. It is of interest that the abnormal right dominance was confirmed by analysis of other brain areas. Autistic patients strongly activated the right middle frontal gyrus but not the comparison subjects, and the difference between both groups was statistically significant. Furthermore, the posterior part of the left middle temporal gyrus, a region that is involved in word processing
(12), was significantly less activated in autistic patients than in the comparison group. This temporal region is also presumed to act as an interface between word perception and long-term representations of familiar words in memory
(13).
These speech-like stimuli elicited an abnormal global cortical mapping in autistic patients characterized by hypoactivation of the left temporal word processing network and also by an emergence of an abnormal right frontotemporal network. Right-biased cortical processing has been previously reported in autism with other types of auditory stimuli
(5,
6). In healthy subjects, the right middle frontal gyrus is activated by an auditory attentional task, i.e., discrimination of sound duration
(14). Therefore, greater activation in the right middle frontal cortex, a region that integrates a cortical “attentional network,” may be implicated in the abnormal and often unexplained behavioral responses to sounds that are one of the most pronounced signs in autism.
The right shift of the global cortical mapping of auditory stimuli and reduced recruitment of the left temporal region in response to complex sounds in autism may be implicated in the abnormal behavioral responses to sounds and language acquisition impairment, both characteristic of autism. As auditory stimuli are perceived as strange electronic noises but never as speech, we speculate that the observed abnormal pattern of auditory activation in autism may reflect a fundamental alteration of the auditory cortical processing that leads to an abnormal early stage of language development rather than reflecting the consequences of abnormal language development. In addition, a similar right-shift pattern of activation was described by Muller et al.
(6) in autistic men listening to human speech sounds. Our results are also in accordance with auditory evoked potential findings in autistic patients of a right hemispheric dominance in the processing of verbal and nonverbal auditory stimuli
(15).
In conclusion, these preliminary results extend to the auditory domain previous findings of abnormal visual cortical pattern of activation in patients with autism
(16–
18), suggesting a disorganization in the establishment of global cortical networks.