Abnormalities in temporolimbic structures have been widely implicated in the pathophysiology of schizophrenia
(1). Postmortem studies investigating the hippocampus in patients with schizophrenia have reported pyramidal cell disarray
(2), reduced pyramidal cell density
(3) and number
(4), smaller neurons
(5), and reduced volume
(6,
7). In contrast, there has been less convincing postmortem evidence for amygdala abnormalities in schizophrenia
(8–
10). Although postmortem studies have provided important neurohistological findings regarding pathology in schizophrenia, potential limitations of such studies include cause of death, illness duration, and extensive prior pharmacologic intervention.
In vivo magnetic resonance imaging (MRI) studies have provided further evidence that patients with schizophrenia have temporolimbic abnormalities. A meta-analysis of 18 volumetric structural MRI studies reported a 4% reduction in bilateral hippocampus volume in patients
(11). Inclusion of the amygdala in this analysis significantly increased the effect sizes across studies, suggesting that schizophrenia involves amygdala pathology as well. Moreover, subsequent MRI studies have also identified hippocampal
(12–
18) and amygdala
(13,
19) abnormalities in schizophrenia, although negative findings have also been reported
(20,
21).
Functional magnetic resonance imaging
(22) and proton magnetic resonance spectroscopic imaging
(23) studies suggest that the hippocampus may be divided along its rostrocaudal axis and that these regions have different neuroanatomical projections and different functional correlates
(24–
26). There is evidence that the dorsal hippocampus (corresponding to the posterior hippocampus in humans) is involved in spatial learning and memory
(27), supporting the idea that this part of the hippocampus is part of a functional network that is connected with sensory cortical areas, including the parietal cortex
(28,
29). In contrast, animal studies suggest that the ventral (corresponding to the anterior hippocampus in humans) or rostral hippocampus has strong connections with prefrontal regions
(30–
32). Moreover, overactivity of the ventral hippocampus has been reported to increase dopamine in the nucleus accumbens
(33,
34). Thus, the anterior hippocampus may be relevant to hypotheses regarding the pathophysiology of schizophrenia
(35–
40) and the mechanism of action of antipsychotic agents that ameliorate symptoms associated with the disorder
(41). Moreover, MRI studies have reported abnormalities in anterior hippocampal regions in patients
(42–
47) that are linked to deficits on neuropsychological tests of frontal lobe function
(35,
40,
48,
49), implicating a defect in frontolimbic connectivity in the pathophysiology of schizophrenia.
Although the anterior hippocampus may be relevant to neurobiological models of schizophrenia, prior volumetric studies of the hippocampus-amygdala complex may have methodological limitations that preclude firm conclusions regarding specificity of anatomic pathology. First, few volumetric studies have distinguished the anterior hippocampus from the posterior hippocampus
(12,
21,
35,
40,
50) despite differences in anatomic connectivity and function. If structural abnormalities are more pronounced in the anterior hippocampus, then studies examining the entire hippocampus might fail to detect significant group differences. Second, of several published studies that implicated anterior hippocampal volumetric abnormalities in schizophrenia
(42–
44,
50–52), only the study by Pegues et al.
(50) measured the anterior hippocampus separately from the amygdala. Thus, in the majority of prior studies anterior hippocampal volumes may have included the most caudal part of the amygdala, whereas amygdala volumes may have included the most rostral part of the anterior hippocampus. It should be acknowledged, however, that in the study by Velakoulis et al.
(12) that used two-dimensional shape information, a selective anterior hippocampal deficit in chronic patients was ruled out.
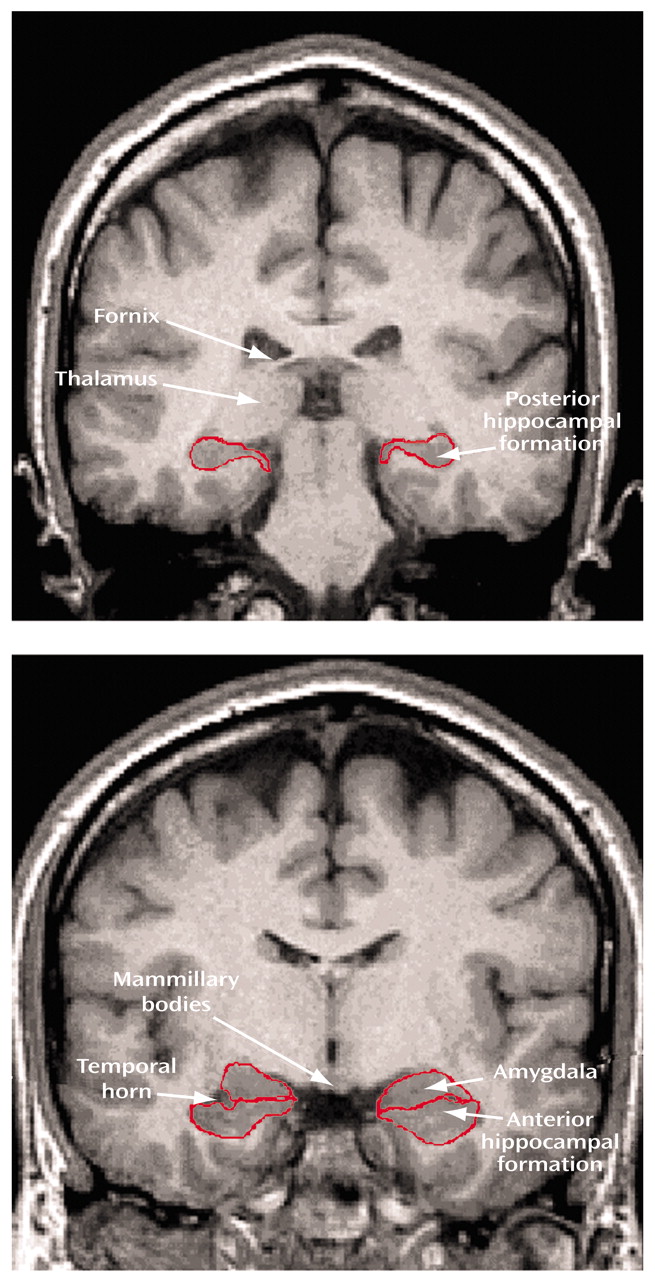
In this study we distinguished between the posterior and anterior hippocampal formation and separated the anterior hippocampal formation from the amygdala. Volumes of these brain regions were computed from contiguous 1.5-mm MRIs in patients experiencing a first episode of schizophrenia and matched healthy comparison subjects. Patients were studied at the onset of their first episode of illness to minimize possible confounds associated with long-term exposure to antipsychotic medications and potential neurodegenerative effects. Moreover, to rule out potential confounds of current antipsychotic exposure on brain structure volumes, a subgroup of patients who had never been exposed to antipsychotic medications also received MRI exams. Given the relevance of the anterior hippocampus to the pathophysiology of schizophrenia, we tested the hypothesis that patients with schizophrenia would demonstrate smaller anterior hippocampal formation volume relative to healthy comparison subjects.
Results
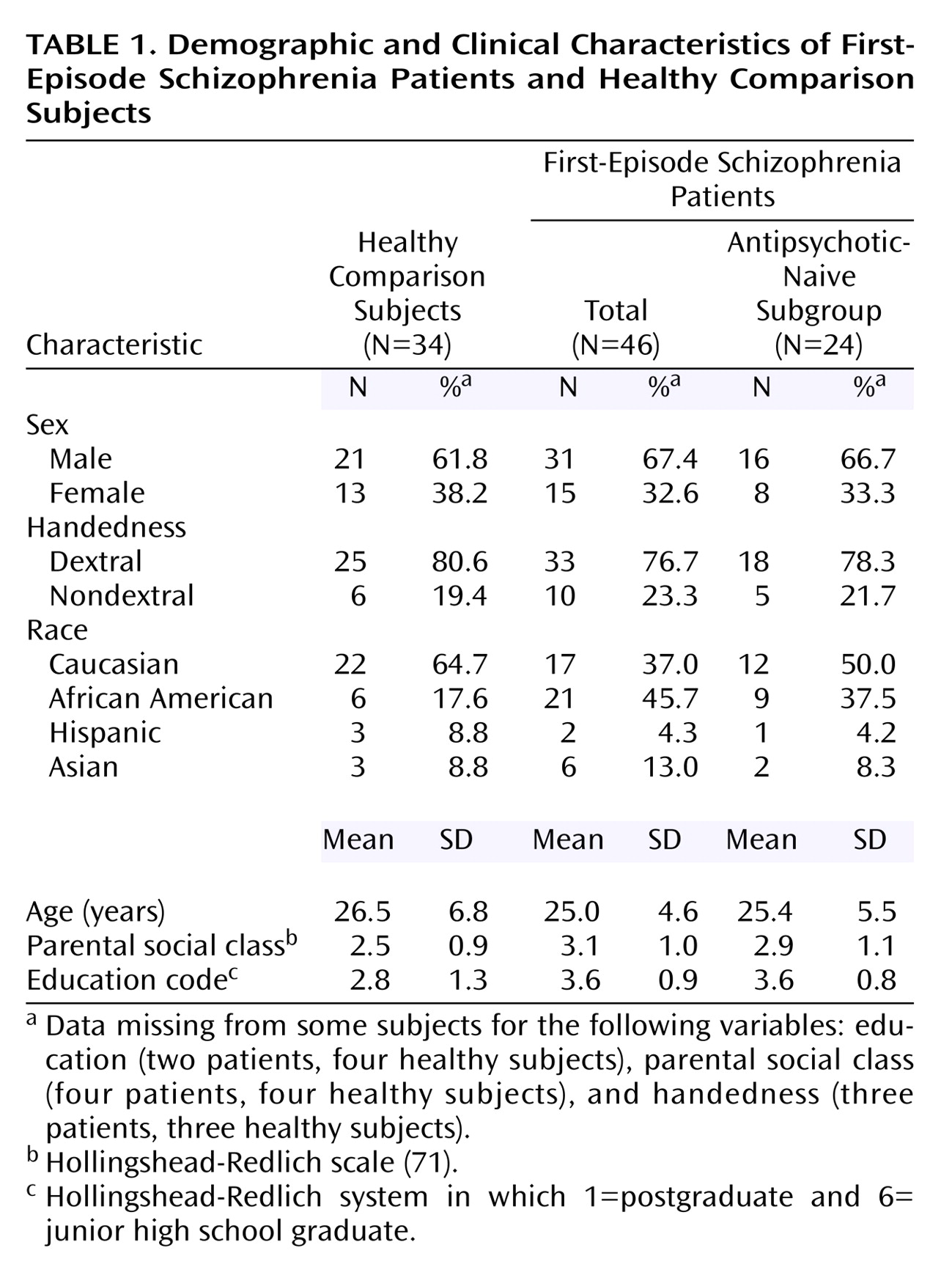
Sample characteristics for the schizophrenia patients and healthy comparison subjects are provided in
Table 1. The entire group of first-episode patients did not differ significantly from the healthy subjects in age, sex, or handedness but did differ in racial/ethnic composition (χ
2=6.03, df=1, p=0.01). In addition, relative to the healthy subjects the schizophrenia patients had a significantly lower parental social class (χ
2=4.70, df=1, p=0.03) and, as expected, less education (χ
2=8.52, df=1, p=0.01). The subgroup of antipsychotic-naive patients did not differ significantly, however, from the healthy subjects in age, sex, handedness, racial/ethnic composition, or parental social class.
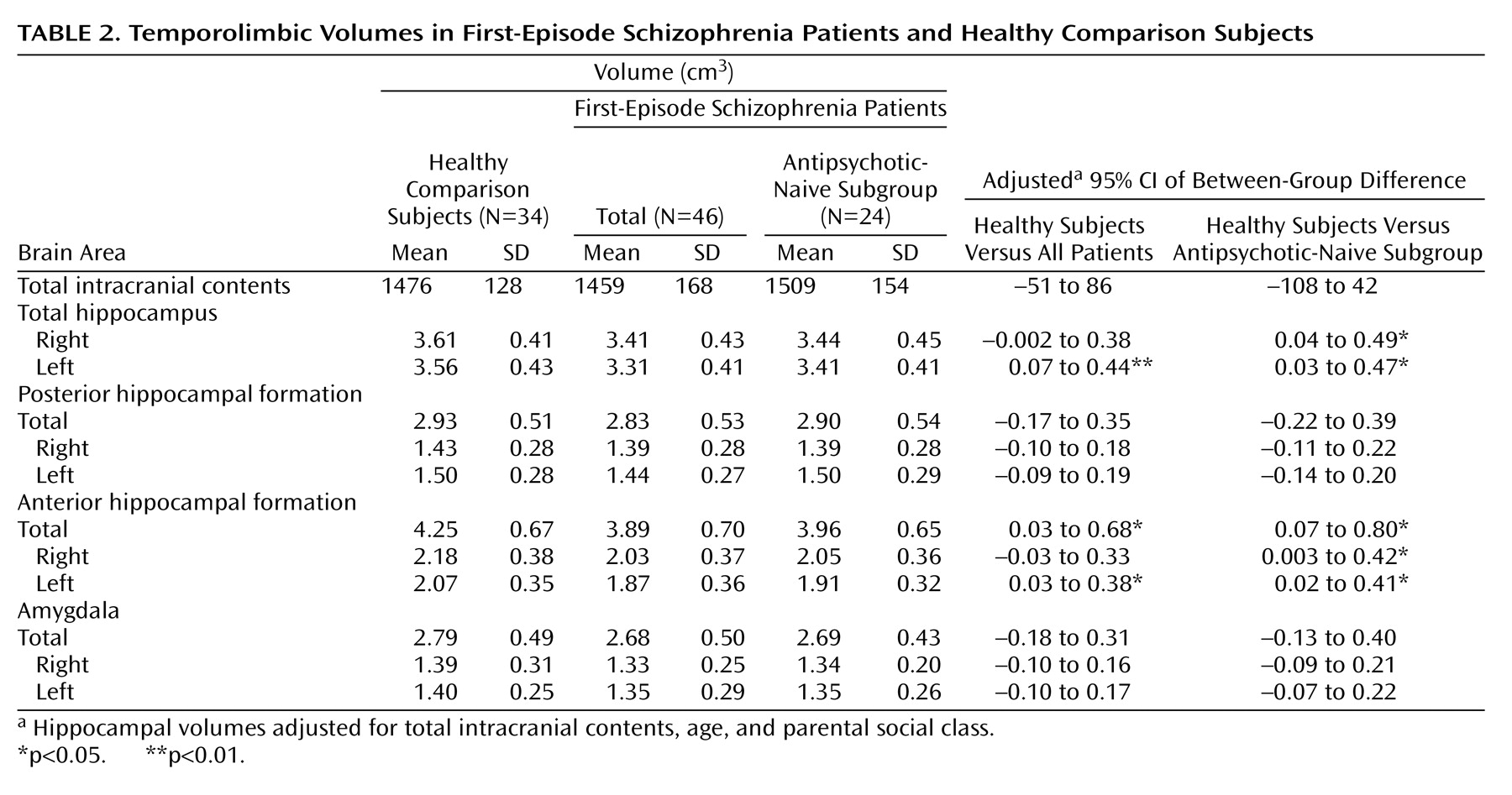
Mean temporolimbic structure volumes for the healthy comparison subjects, the entire group of first-episode schizophrenia patients, and the antipsychotic-naive subgroup of patients are presented in
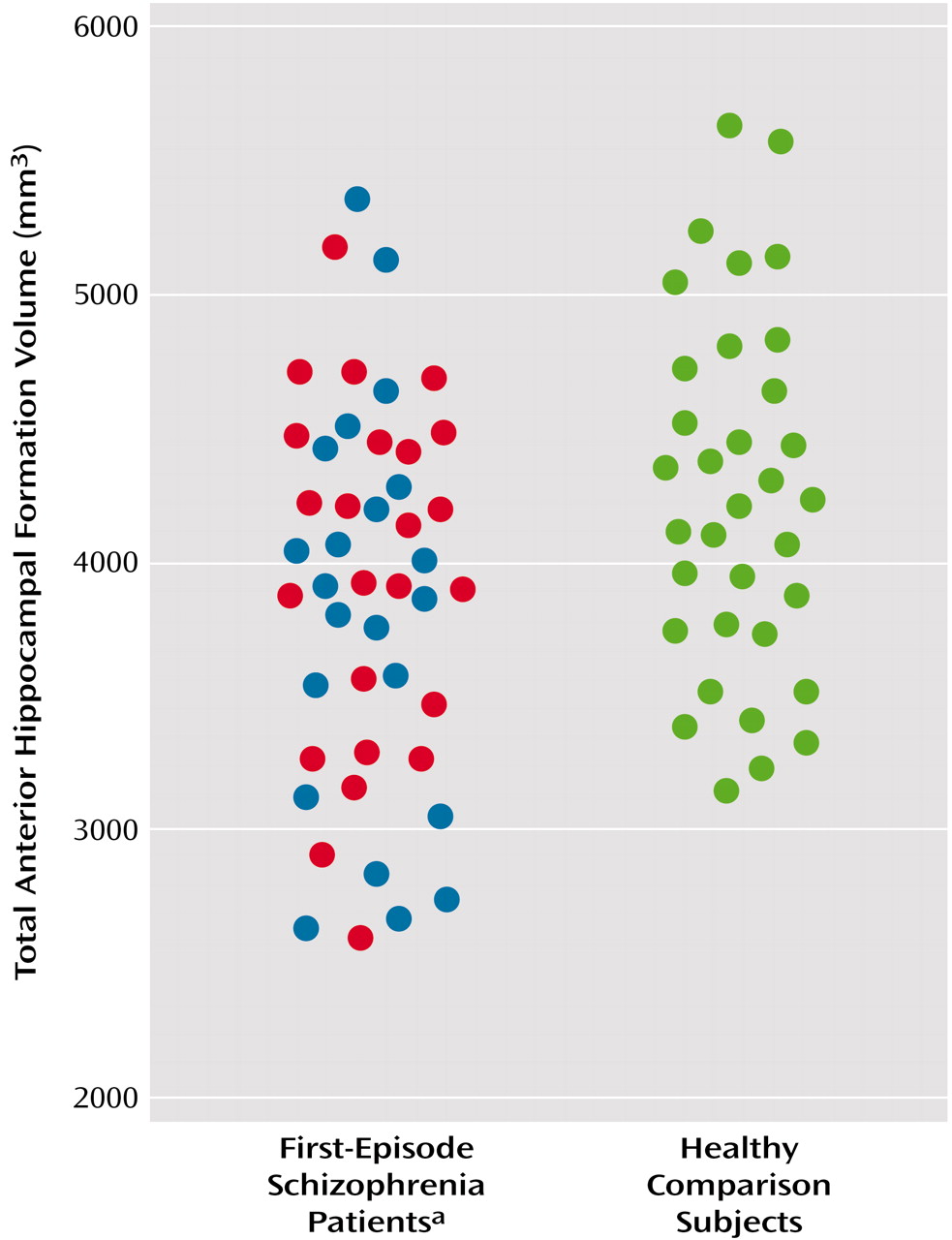
Table 2, along with the adjusted 95% confidence intervals for the differences between group means. Analyses performed for the entire group of first-episode patients revealed significantly smaller total volume of the anterior hippocampal formation compared with the healthy subjects (F=5.21, df=1, 76, p<0.03), which remained significant after the effects of age, parental social class, and intracranial volume were controlled (F=4.47, df=1, 65, p<0.04). Total volume of the anterior hippocampal formation is illustrated for patients and healthy subjects in
Figure 2. Given the ethnic/racial differences between the overall patient sample and the healthy subjects, we analyzed the subgroup of Caucasian subjects (the only subgroup large enough for analysis) separately, which confirmed the original finding of smaller anterior hippocampal formation volume in patients (F=5.06, df=1, 28, p<0.04). The main effect of sex was significant for the anterior hippocampal formation (F=6.80, df=1, 76, p=0.01) and amygdala (F=6.85, df=1, 76, p=0.01) such that male subjects had larger volumes of these structures overall than did female subjects. After we controlled for the covariates, however, the main effect of sex remained statistically significant only for amygdala volume (F=4.37, df=1, 65, p<0.05). Neither the group-by-sex nor group-by-hemisphere interactions were statistically significant for any of the temporolimbic structure volumes in analyses either with our without the statistical covariates.
Additional analyses investigated the possible effects of antipsychotic treatment on these findings by excluding those patients from analyses who had been exposed to antipsychotic medications. Antipsychotic-naive patients also demonstrated significantly smaller volume of the anterior hippocampal formation relative to the healthy subjects (F=6.23, df=1, 43, p<0.02) (
Table 2). In addition, similar to the findings observed for the overall patient sample, there were no significant group volume differences for either the posterior hippocampal formation or amygdala. Total volume of the anterior hippocampal formation did not correlate significantly with any of the clinical measures or duration of psychotic symptoms before study entry in either the overall sample of patients or the antipsychotic-naive subgroup of patients.
Discussion
Although the anterior hippocampus is highly relevant to several neurobiological models of schizophrenia, many neuroimaging studies have not distinguished this region from the posterior hippocampus or amygdala, making it difficult to address issues regarding specificity of anatomic pathology. To our knowledge, this study provides the first evidence of volumetric alterations in the hippocampus-amygdala complex that are specific to the anterior hippocampal formation in antipsychotic-free patients experiencing a first episode of schizophrenia.
Our findings are consistent with and extend prior MRI evidence of anterior hippocampal-amygdala volume reductions in schizophrenia. Shenton et al.
(42) and Rossi et al.
(46) reported smaller anterior hippocampus-amygdala volume in patients with schizophrenia compared with healthy subjects. Suddath et al.
(43) found reduced temporal lobe gray matter among patients in a central temporal lobe subdivision that anatomically corresponded to the anterior hippocampus and amygdala. A subsequent study by Suddath et al.
(44) found that the right and left pes hippocampi were smaller in the affected twin of monozygotic twin pairs discordant for schizophrenia compared respectively to their normal twins. Our results also converge with the recent study by Pegues et al.
(50), who reported that anterior hippocampal volume was smaller in older (mean age=35.1 years) male patients who had been treated with antipsychotics compared with healthy male subjects.
In contrast to other reports indicating that smaller hippocampus volume was lateralized to the left hemisphere
(42–
44) or more pronounced in male than in female patients
(51,
52), we did not find evidence for either effect. Our findings are consistent, however, with Pegues et al.
(50), who also did not identify a selective left hemisphere deficit in their sample of male patients. Sampling and methodologic differences in defining the hippocampus and amygdala may account for these discrepant findings, thus making it difficult to directly compare studies. For example, in some of our prior work
(35,
40) we did not separate the anterior hippocampal formation from the amygdala but instead used a midline landmark (i.e., the mamillary bodies) to distinguish between these regions.
The finding of smaller anterior hippocampal formation volume in patients may be consistent with neurodevelopmental
(72) or postnatal influences on hippocampal morphology
(73,
74). Specifically, abnormal pre- and postnatal hippocampal development has been found to also be associated with factors such as genetic variants
(75), viral infection
(76), stress
(77), or obstetric complications
(78). Although neurodegenerative mechanisms cannot be entirely ruled out, there was no association between volume of the anterior hippocampal formation and duration of psychotic symptoms before study entry, which argues against this possibility. It should be acknowledged, however, that such an association might be observed during the course of illness or in more chronic patients.
An abnormality involving the medial frontolimbic system or dorsal “archicortical” trend has been hypothesized to comprise, at least in part, the structural basis for frontal lobe dysfunction in schizophrenia
(35,
40,
48,
79). Consistent with this hypothesis are findings from MRI studies investigating the functional sequelae of hippocampal pathology in schizophrenia, which have more often implicated the anterior hippocampus than the posterior hippocampus. Weinberger et al.
(49) found that differences in anterior hippocampus volume computed between monozygotic twins discordant for schizophrenia correlated with the differences between the twins in regional cerebral blood flow to the prefrontal cortex during performance of the Wisconsin Card Sorting Test. Moreover, reduced volume in the anterior (but not posterior) hippocampal formation was found to be significantly correlated with lower scores on neuropsychological tasks considered sensitive to the integrity of frontal lobe functions in schizophrenia
(35), with this effect being more pronounced among male patients in a larger sample
(40).
Findings from animal studies also suggest that early developmental lesions to the hippocampal formation may yield both pharmacologic and behavioral abnormalities consistent with frontal lobe lesions in adult animals
(80–
83). In particular, an excitotoxic lesion to the rat ventral hippocampal formation (corresponding to the anterior hippocampal formation in humans) produced increased mesolimbic dopamine responsiveness to stressful stimuli along with deficits in socioemotional functions
(80,
81) and altered the development of neural circuits mediating certain dopamine and
N-methyl-
d-aspartic acid-related behaviors
(83). Of interest is that these abnormalities became apparent in these animals only upon maturation into adolescence or adulthood. Extrapolating from the animal literature, it is therefore conceivable that a neurodevelopmental defect involving the anterior hippocampal formation could yield a pattern of frontal lobe dysfunction in schizophrenia through a disruption in frontolimbic connectivity
(35,
40).
There are several limitations to this study that preclude firm conclusions. It is important to acknowledge that more subtle abnormalities may be present in the posterior hippocampal formation or amygdala in patients that were not detected using these volumetric methods. For example, some studies that analyzed the shape of the hippocampus-amygdala complex reported abnormalities in the posterior hippocampus
(12) and amygdala
(19). Thus, including additional information regarding shape in analyses may improve group discrimination
(18,
84). In addition, although consistent with functional neuroimaging data, our use of the cisterna pontis to distinguish between the posterior and anterior hippocampal formation should not be interpreted to enable a strict subdivision of the hippocampal rostrocaudal axis. Future cytoarchitectonic and functional neuroimaging studies might better clarify a subdivision of the hippocampus along the rostrocaudal axis.
In summary, we report volumetric alterations of the anterior hippocampal formation in patients experiencing a first episode of schizophrenia in the absence of group volume differences for the posterior hippocampal formation or amygdala. In vivo studies conducted at higher field strengths may be able to resolve the internal architecture of the hippocampus-amygdala complex to more precisely determine the nature of purported anterior hippocampal pathology in schizophrenia.