No drugs have been shown to consistently improve the social and communication impairments central to autism. Open-label studies of serotonin reuptake inhibitors and atypical antipsychotics have suggested improvement in certain aspects of social relatedness, but this has yet to be proven in placebo-controlled trials. In a previous randomized, placebo-controlled study of 101 children with autism and severe irritability
(1), risperidone led to significant improvement in irritability, hyperactivity, and stereotypic behavior, but not in social withdrawal or inappropriate speech. Thus, treatments for the core social and communication impairments that characterize autism are needed.
Several authors have drawn parallels between the negative symptoms of schizophrenia and the social and communication impairment observed in autism. Low-dose
d-cycloserine, a partial agonist at the
N-methyl-
d-aspartate (NMDA) glutamate receptor subtype, has been shown to reduce negative symptoms in adults with schizophrenia
(2). Drugs affecting the glutamate system have begun to be explored as therapeutic agents in several neuropsychiatric disorders
(3) but have received limited attention as a possible therapeutic option in autism. Carlsson has hypothesized that autism is a hypoglutamatergic disorder on the basis of NMDA antagonist effects in humans and mice
(4). With these points in mind, we undertook a pilot study of
d-cycloserine to determine whether it had any short-term clinical benefits or adverse effects in subjects with autism.
Method
Subjects (age 5 and older) with autistic disorder (meeting DSM-IV and Autism Diagnostic Interview—Revised criteria) were eligible for this study, which was approved by the Indiana University Institutional Review Board. The study was completely described to both the subject and a parent; after this, written informed consent and assent (in the case of subjects less than 18 years old) were obtained. Twelve drug-free subjects with autism were enrolled in the study.
The study was prospective and single-blind in design. Following a 2-week placebo lead-in phase, d-cycloserine was administered in ascending doses of approximately 0.7, 1.4, and 2.8 mg/kg/day, each for 2 weeks. d-Cycloserine was administered using identically appearing capsules containing either 25 mg of drug or placebo.
Ratings were done at baseline and at the end of each 2-week phase of the 8-week study. Ratings included the Clinical Global Impression (CGI) scale, the Social Responsiveness Scale, a modified Children’s Yale-Brown Obsessive Compulsive Scale, and the Aberrant Behavior Checklist. The Aberrant Behavior Checklist is a 58-item parent-rated instrument with five subscales (irritability, social withdrawal, stereotypic behavior, hyperactivity, and inappropriate speech). The social withdrawal subscale consists of 16 items, each of which can be rated from 0 (not a problem) to 3 (severe problem).
Subjects with CGI improvement ratings of “much improved” or “very much improved” during the placebo lead-in phase were to be withdrawn from the study. Height, weight, vital signs, and adverse events were recorded at all visits. A physical examination, laboratory measures, and an electrocardiogram were done at baseline and at the conclusion of the study.
The baseline ratings and placebo ratings were combined and served as a new baseline for the statistical analyses. Outcome measures were analyzed by using a repeated measures analysis of variance. Post hoc comparisons were done by using Dunnett’s procedure. Response to treatment was defined as a CGI improvement rating of “much improved” or “very much improved.” Results are reported as means and standard deviations. All statistical tests are two-tailed with significance set at p<0.05.
Results
Two subjects withdrew from the study after completing only the 2-week placebo lead-in phase. One of these subjects dropped out because of worsening stereotypic behavior, and the other subject dropped out because of noncompliance. The remaining 10 subjects (eight male and two female subjects; mean age=10.0 years, SD=7.7, range=5.1–27.6) completed all 8 weeks of the study and were included in the final data analysis. The mean daily doses of
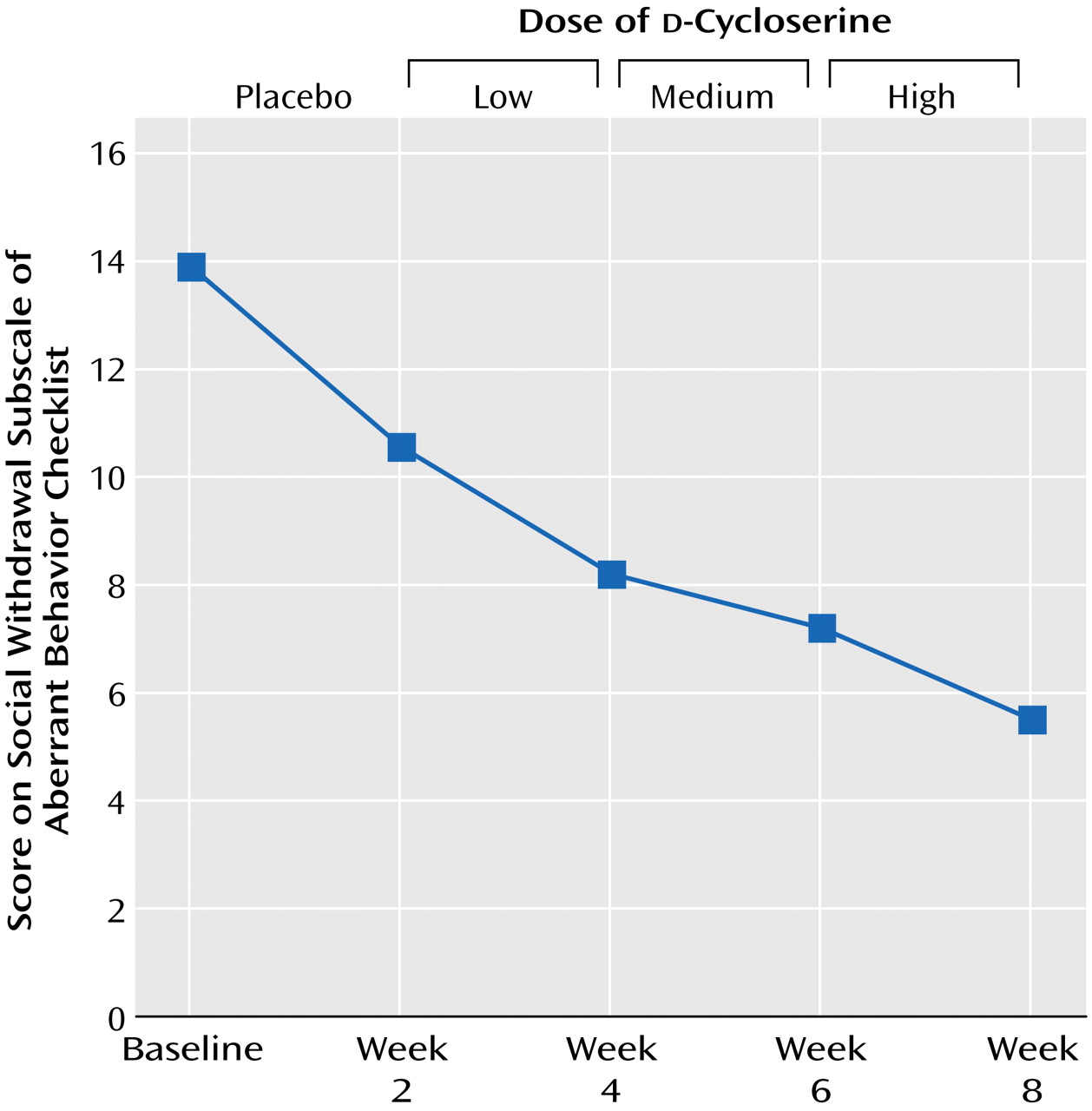
d-cycloserine at each of the three dose levels (low, medium, high) are shown in
Figure 1.
d-Cycloserine was associated with a statistically significant improvement in the CGI severity rating (F=4.58, df=4, 43, p=0.007). A post hoc comparison indicated that ratings following treatment with both the medium (p=0.02) and high doses (p=0.008) of d-cycloserine were significantly different from baseline. For CGI improvement ratings, response rates for the placebo, low-dose, medium-dose, and high-dose phases were 0%, 30%, 40%, and 40%, respectively.
A statistically significant improvement was also seen on the Aberrant Behavior Checklist social withdrawal subscale (
Figure 1). A post hoc comparison indicated that scores following treatment with the highest dose of
d-cycloserine were significantly different from those at baseline (p=0.02). At this dose, there was a 60% decrease in symptom severity. There were no significant differences on the other Aberrant Behavior Checklist subscales (irritability, stereotypic behavior, hyperactivity, inappropriate speech), Social Responsiveness Scale, or Children’s Yale-Brown Obsessive Compulsive Scale.
Two subjects experienced adverse effects (a transient motor tic and increased echolalia) at the highest dose they received (2.8 and 3.0 mg/kg/day, respectively). End-of-study physical examination results and laboratory values were not significantly different from pretreatment.
Discussion
In this preliminary study, d-cycloserine treatment was associated with reduced social withdrawal and increased social responsiveness in a group of subjects with autism. In 40% of these subjects, the improvement was clinically meaningful, and both the parent and clinician rated them as “much improved.”
Relative strengths of this study include the use of well-characterized drug-free subjects and the prospective, placebo-controlled, single-blind design. Limitations include the wide age range, lack of clinician blinding, and the absence of a parallel control group. Given the small number of subjects in this exploratory study, it is likely that a type II error exists. Indeed, the means of several of the outcome measures improved during d-cycloserine treatment but failed to reach the level of statistical significance. This raises the possibility that some of the improvement seen during the study was due to the effects of d-cycloserine on related symptoms of mood disturbance or behavior.
In addition, because the order of treatments was not randomized, we cannot rule out that the effects seen at the higher doses of
d-cycloserine were secondary to a delayed placebo effect or to longer duration of treatment with lower doses of
d-cycloserine. This is also important because it is possible that the adverse effects seen during the highest dose of
d-cycloserine were due to longer duration of treatment. Furthermore, because a full range of
d-cycloserine doses was not tested in this study, it is possible that the optimal dose of
d-cycloserine may lie in between those specifically examined. Finally, it is possible that some of the clinical effects seen in this study are due to NMDA receptor antagonism rather than agonism, since
d-cycloserine is a partial agonist with different affinities for NMDA receptor subtypes
(5).
Overall, these preliminary results suggest that d-cycloserine might have efficacy for certain aspects of social impairment in autism. A randomized controlled trial of d-cycloserine in autism appears warranted.