Mismatch negativity is an event-related potential elicited when a sequence of unattended repetitive sounds is interrupted by a deviant stimulus
(1). It is an electrophysiological correlate of sensory memory representation and can be observed even under distracted conditions. This preattentive response is generated at the level of the planum temporale and is likely to reflect
N-methyl-
d-aspartic acid (NMDA) channel current influx in cortical layers II and III as found in extracellular recordings in the monkey
(2). In humans, a diminished mismatch negativity response following the administration of ketamine (an NMDA antagonist) has been described
(3), consistent with NMDA/glutamate involvement in mismatch negativity generation.
The mismatch negativity response to duration deviants, usually recorded with EEG, is consistently reduced in patients with schizophrenia
(4–
7). In some studies of pitch deviants, this abnormality was not observed
(8,
9) (for a review see reference
10). This impairment of sensory memory formation has also been described in unmedicated patients
(4), first-degree relatives
(11,
12), and in high-risk groups
(13). Several studies have reported correlations between mismatch negativity amplitude and negative symptoms
(4,
14), and it appears that the mismatch negativity response is particularly reduced over the left hemisphere in patients with schizophrenia
(15–
17). The mismatch negativity reduction seems not to be ameliorated by either typical
(18) or atypical neuroleptics
(19).
Mismatch negativity response in schizophrenia is of particular interest given the well-replicated findings of structural superior temporal gyrus abnormalities
(20,
21) and the involvement of this brain area in core symptoms of the disease such as hallucinations
(22,
23) and formal thought disorder
(24). Furthermore, NMDA is suspected to be involved in the pathophysiology of schizophrenia
(25). However, little is known about the mechanisms of decreased mismatch responses in schizophrenia, their etiological significance, or their precise locus of generation.
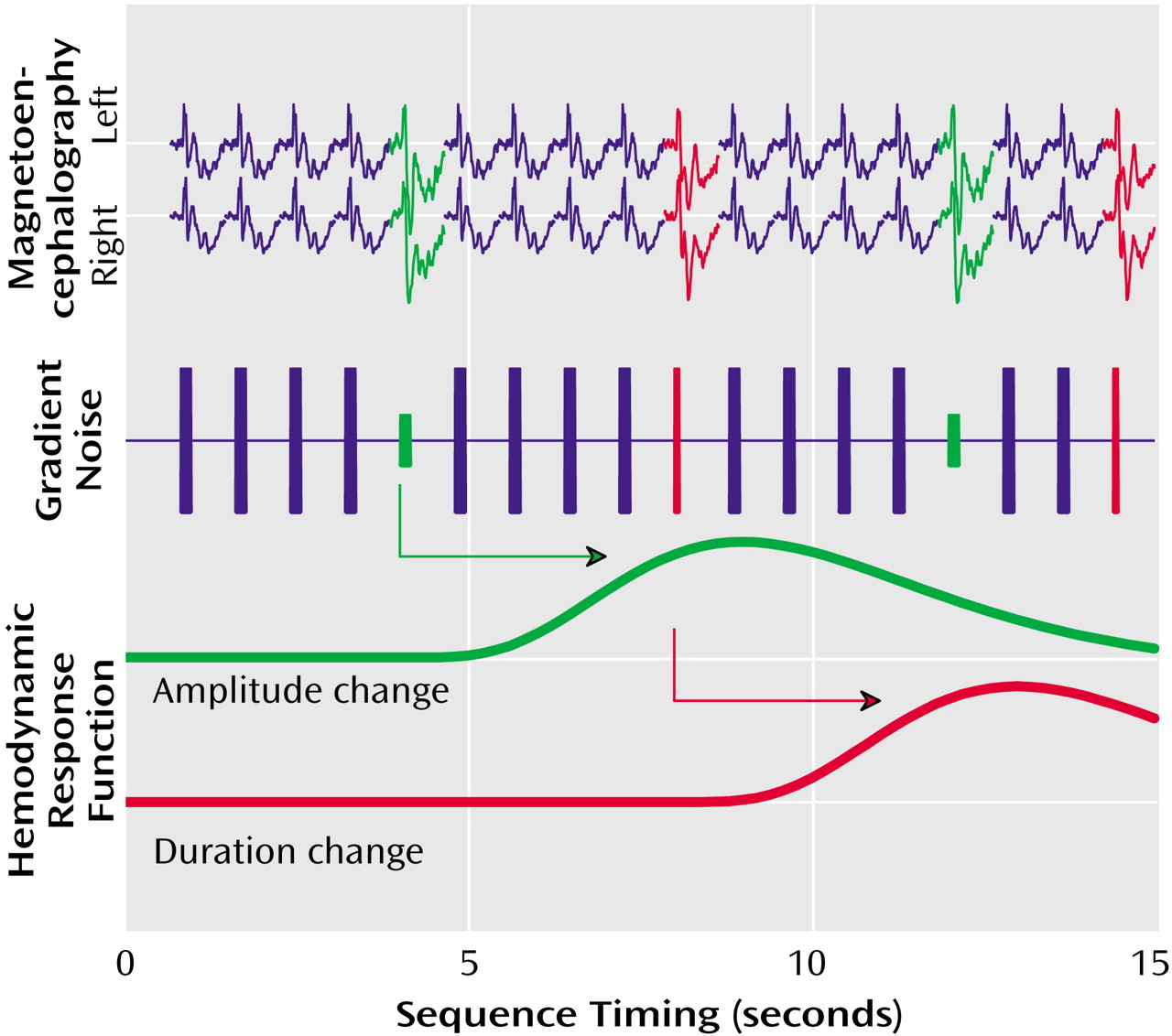
Functional magnetic resonance imaging (fMRI) allows precise localization of cerebral correlates of perceptual and cognitive operations. However, the noise generated by the scanner limits its application in the investigation of auditory processing. To avoid these shortcomings, we developed a method to record the hemodynamic analogue of mismatch responses by means of fMRI using the gradient switching noise as the sound source
(26). The advantage of this technique is—besides its ease of use without external stimulation devices—that the sound source is part of the environment so that unattended processing prevails. This stimulation procedure does not yield the frontal activation
(26) that suggests attentive processing as observed with other fMRI or positron emission tomography techniques. Moreover, the scanner noise can be recorded and applied within whole-head magnetoencephalography (MEG) to obtain comparable electrophysiological data.
Discussion
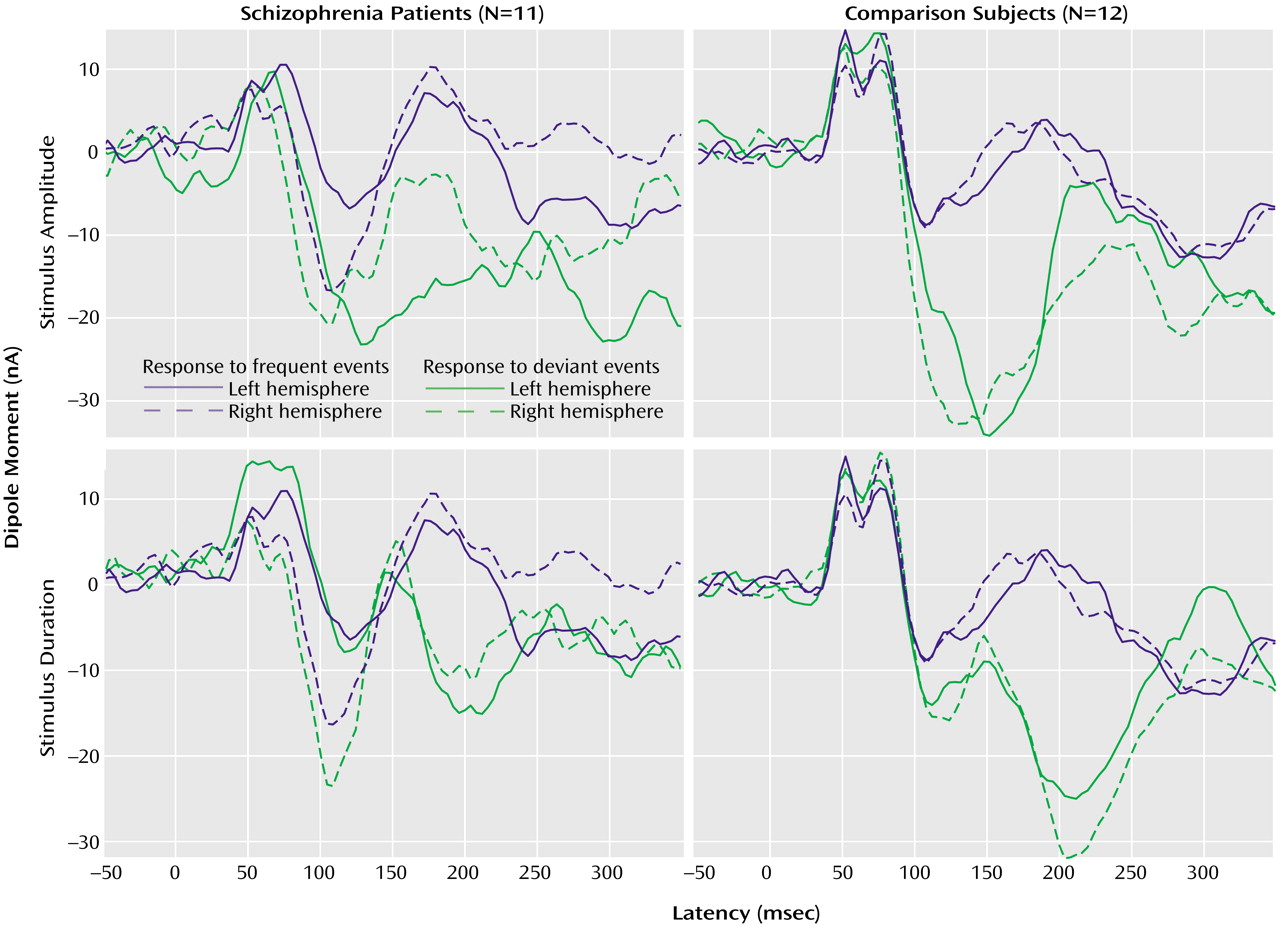
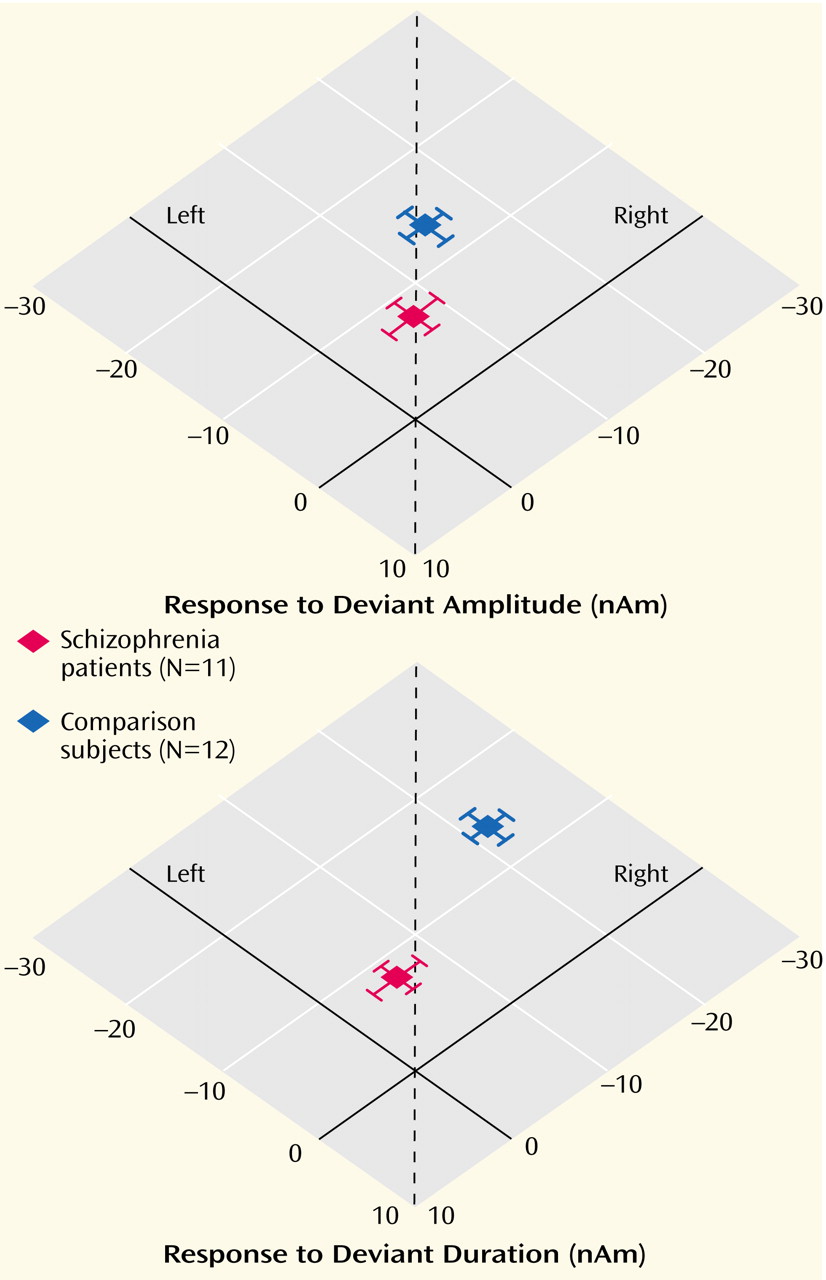
This study employed a novel design in a combined fMRI and MEG setting to compare cerebral responses to deviant auditory stimuli in patients with schizophrenia and matched comparison subjects. To circumvent the interfering background sound of the MRI scanner, its gradient switching noises were used as stimuli. The noise was recorded and applied in a whole-head MEG device. In the comparison group, the deviant stimuli evoked reliably right-lateralized mismatch MEG responses. The patients exhibited decreased responses and failed to show a lateralization effect in line with our hypothesis. In the fMRI setting, duration deviants elicited right lateralized signal increases in the planum temporale in the comparison subjects. The patients, in contrast, exhibited symmetric signal changes. The results of this study confirmed our earlier findings in the comparison subjects
(26), and the patient data confirms our hypothesis of dysfunctional lateralization in this group.
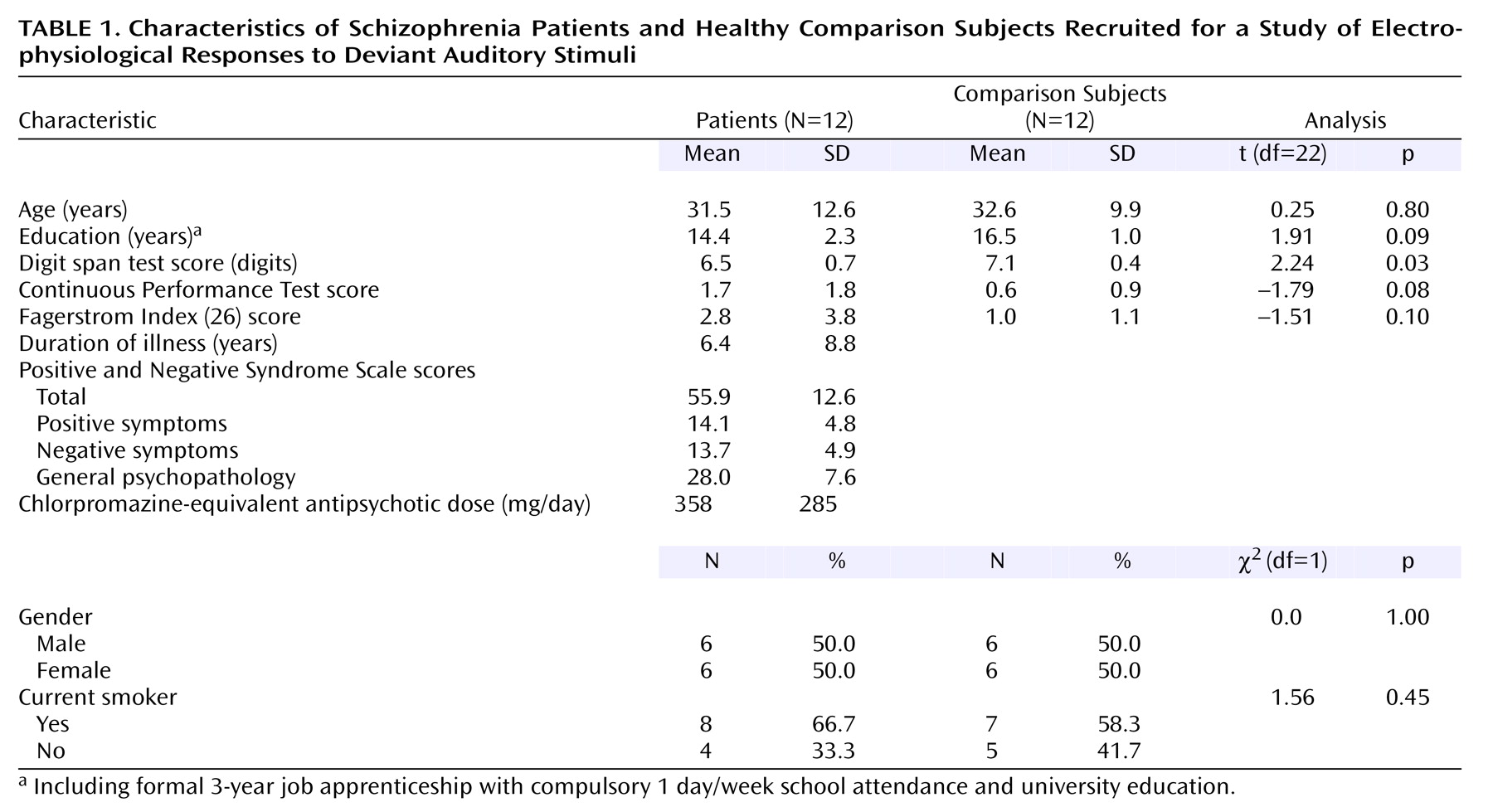
The groups in our study were carefully matched for possible confounding effects such as age, gender, and nicotine use. Previous event-related potential studies have reported effects of smoking on P50 and P300
(41,
42), although influences on N100 have not been reported. Neuroleptic medication does not seem to influence mismatch negativity response
(18,
19). Because of the size of our study group, the influence of gender on lateralization could not be calculated.
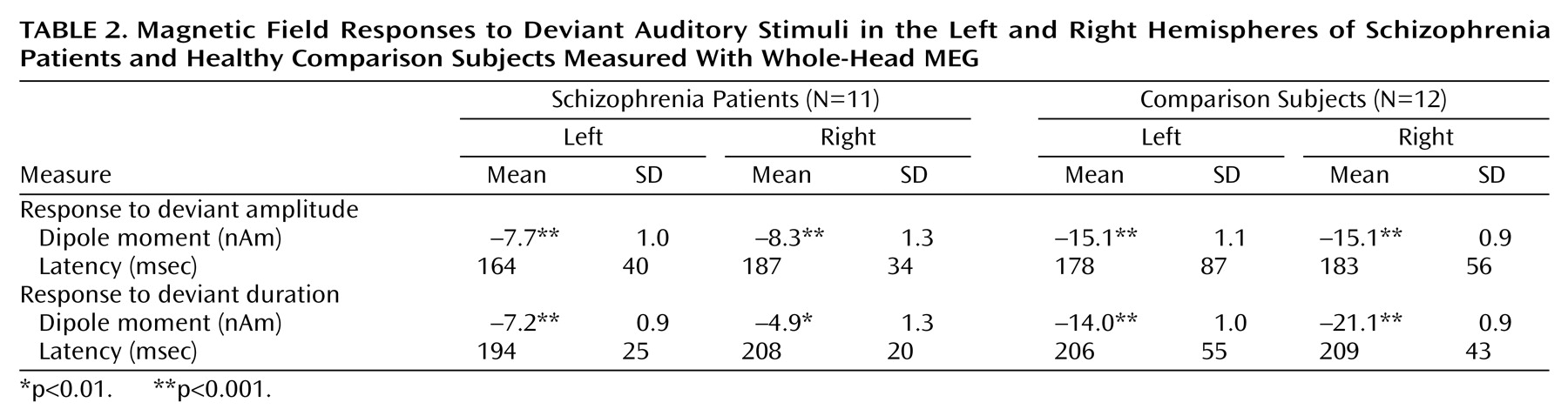
Although our mismatch stimuli might be slightly different in interstimulus interval or duration from standard EEG paradigms, they reliably evoked the well-known neuromagnetic responses in different control populations
(26). Corroborating earlier findings of EEG studies
(36,
43), the patients showed reduced mismatch fields to the duration as well as amplitude deviants. We could extend these findings and demonstrate that the decrease is at least in part explained by the missing right-hemispheric enhancement in the patients in the planum temporale, but not Heschl’s gyrus. In the only other MEG study investigating mismatch field in schizophrenia
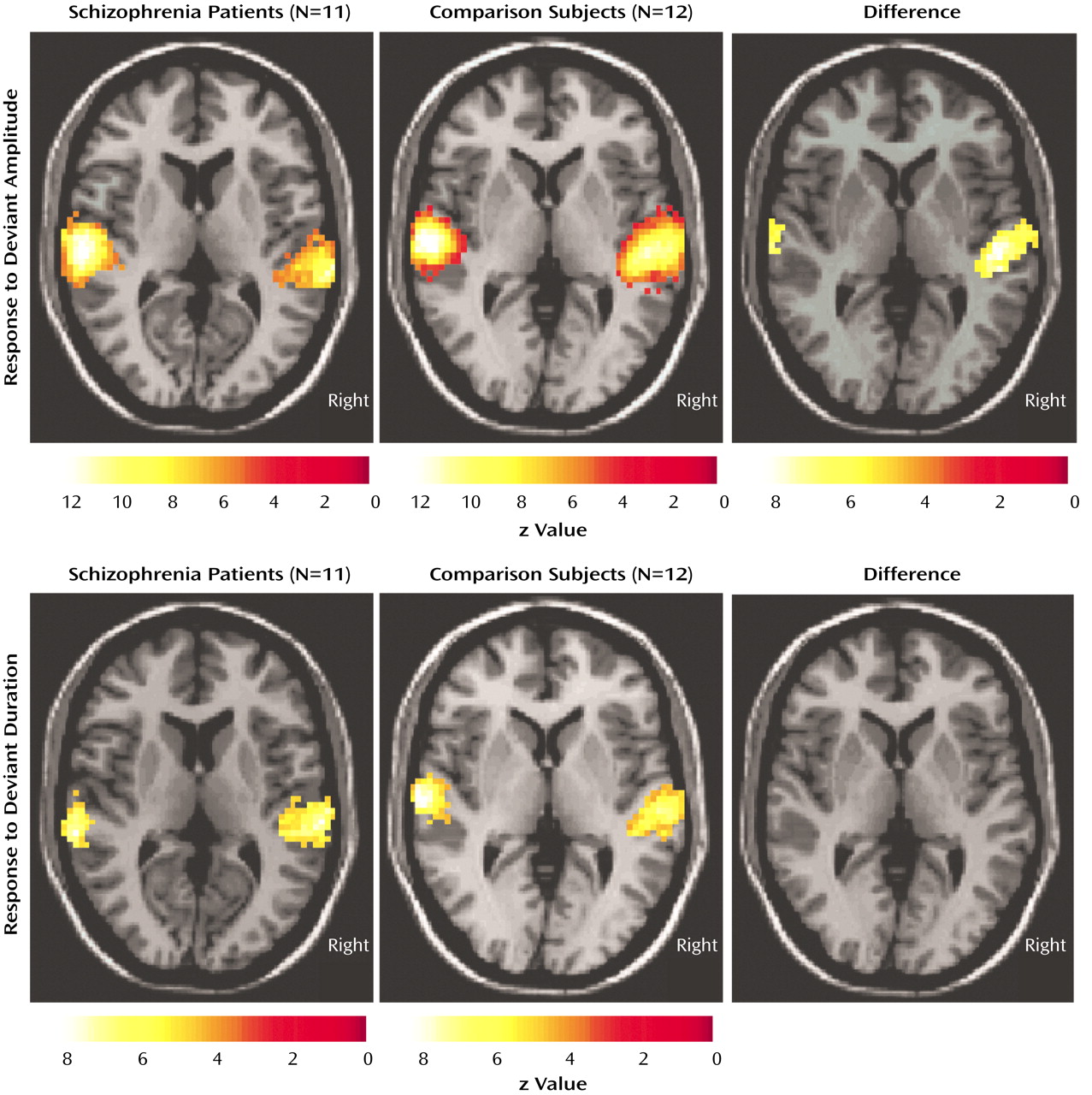
(16), such an altered lateralization was not present. However, those authors did not find the normal lateralization pattern to the right in comparison subjects. They used a MEG device that could record responses over one hemisphere only. The sequential comparison of the responses might increase variance and be influenced by a crosstalk from the unobserved auditory cortex. We used a whole-head scanner, which is more reliable in detecting lateralization differences.
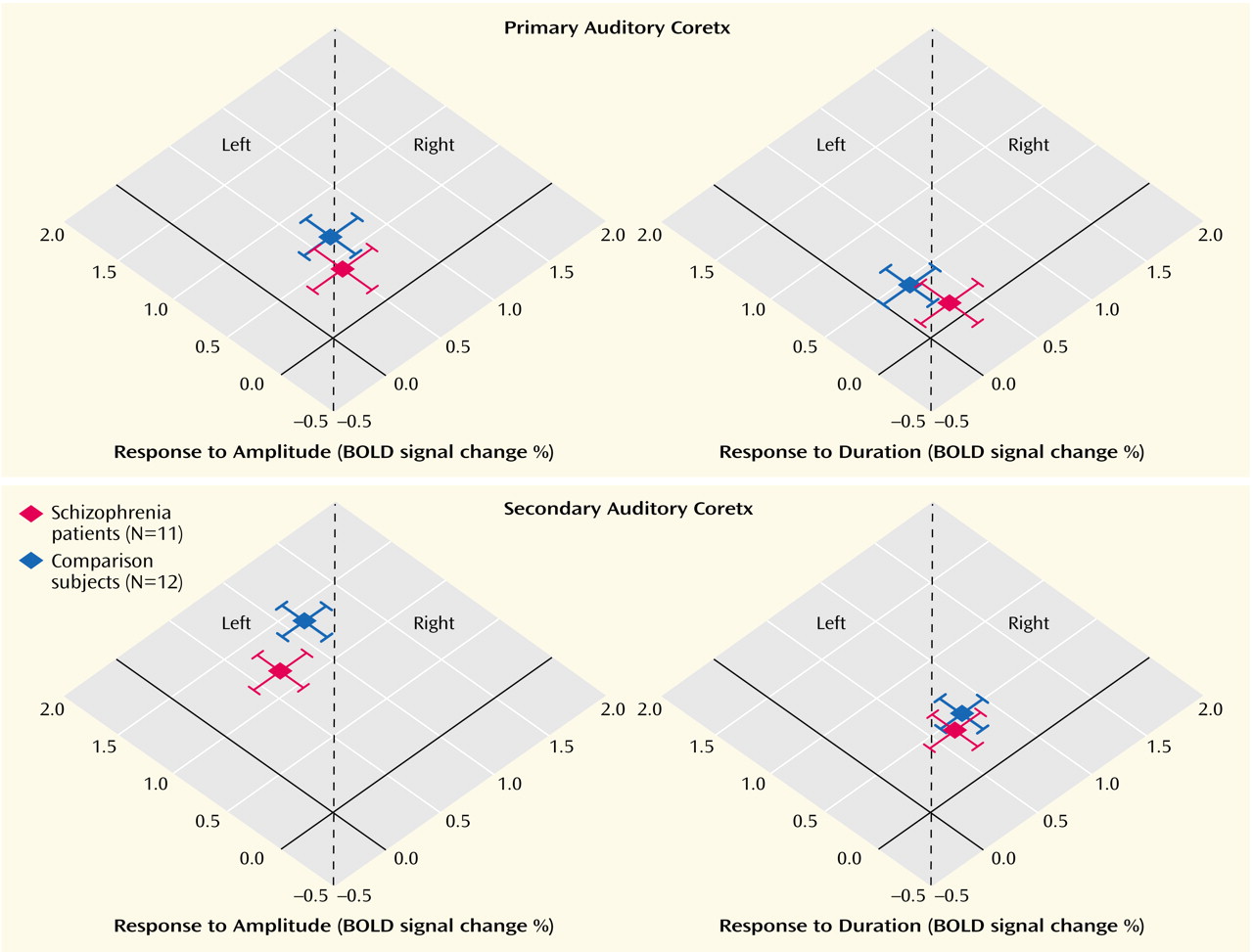
Previously, it has been demonstrated that the mismatch negativity response has multiple generators within the superior temporal gyrus
(44). The precise localization of its main component cannot be detected with current MEG methods. Using fMRI with its high spatial resolution, our results demonstrated that the hemodynamic equivalent of the mismatch field in the comparison subjects is in part located in the secondary auditory cortex (planum temporale), with a stronger signal change in the right superior temporal plane. Furthermore, a lateralization deficit similar to the mismatch field paradigm in the patients for the duration mismatch was detected. This finding stands in contrast to another fMRI study of mismatch negativity response in schizophrenia, in which standard oddball stimuli were presented via headphones
(45). The authors found a smaller number of activated voxels in the superior temporal gyrus in the patients than in the comparison group in the mismatch but not the control condition. They did not find lateralization differences between groups, and the strongest activation was present in the Heschl’s gyrus (primary auditory cortex) in both groups. However, a confounding effect in their study may have been scanner noise, which is known to activate primary auditory regions
(46,
47) and to mask the effects of stimulus properties. Another difference is that they employed blocks with deviants compared with blocks without deviants whereas we assessed directly the hemodynamic response to the single deviant events. Thus, lateralized abnormality might be masked by the difference in the activations to the blocks without deviants or specific to the distinct types of deviance detection.
The role of frontal areas in the generation of mismatch fields is still under debate. A number of studies found differences in mismatch responses between patients with schizophrenia and comparison subjects in the frontal
(48–
50), in the frontal and temporal
(51,
52), or exclusively temporal
(53–
55) regions. Neither in our comparison nor in the patient group did we find responses in frontal areas. Differences in the technique applied and stimulus properties might be responsible for the different results in these studies. It has been proposed that the involvement of frontal areas in the generation of mismatch fields reflects attentive components
(56,
57). The comparison with event-related potential recordings suggests that distinct networks participate in preattentive processing and target detection
(58). Our paradigm allowed stimulation without attentive processing and thus little frontal contributions would be expected. Moreover, putative attentional deficits in patients would not have an influence in our paradigm. Thus, we focused on the auditory cortices, where we expected the most reliable differences between the groups. Further, our fMRI paradigm allowed only for a limited number of slices to be acquired, and the ventral regions of the frontal lobe were therefore not covered.
The BOLD response reflects differential hemodynamic activation integrated across seconds. Thus, neither early versus late components of a mismatch response, nor processing of a deviant versus its succeeding frequent stimulus, can be entirely separated. Amplitude changes modified responses in the primary auditory cortex due to deviant detection as well as adaptation effects, which cause primary, as well as secondary, auditory cortical responses. The effects of duration deviants on primary areas were minimal. Peak sound pressure level remains the same for the duration deviants whereas amplitude deviants may change the adaptation level. Therefore, the first louder sound may elicit adaptation phenomena in primary auditory areas. We suggest that an integration of activity in the primary and secondary
(59) auditory cortices of both hemispheres is necessary for a normal mismatch response. A temporal desynchronization of the process is likely to underlie the deviant mismatch field response in our patients
(60). The differences in mismatch fields and BOLD response between the
groups in our study might be due to alterations in synaptic connections, pyramidal cell distribution, or connectivity between cortical columns. This might cause reduced stimulus-induced phase locking in the auditory cortex in the patients
(61) and thus altered mismatch fields. The fMRI and MEG experiments yielded distinct effects for the topography and lateralization patterns. As a possible explanation, later components might be integrated into the BOLD response, whereas the mismatch field is restricted to the given temporal domain. Nonetheless, no differences emerged in the temporally resolved MEG for the response latencies between the groups. The different lateralization patterns in the fMRI and MEG data may thus reflect differences in temporal integration and synchronization processes between patients and comparison subjects.
Mismatch negativity elicitation is attributed to the presence of short-term memory traces. Templates are formed in the auditory cortex to encode the frequent stimulus elements. These memory traces usually fade within 5–10 seconds
(62). For mismatch negativity to occur, an integrative process, consisting of an analysis of the afferent sound (deviant) and its comparison to the memory trace (frequent stimulus) has to occur
(63). Impaired language processing in childhood is one of the few known risk factors for the later development of schizophrenia
(64). Furthermore, some of the main symptoms in schizophrenia, such as formal thought disorder, auditory hallucinations, and concretism, involve language functions. We previously demonstrated a reversed lateralization pattern of cerebral activation in the superior temporal gyrus in patients with schizophrenia during processing of linguistic context
(65) and production of fluent speech
(66). The production of thought-disordered speech correlated negatively with signal changes in the posterior superior temporal gyrus (Wernicke’s area
[24]).
In the current study, we have shown that language-related dysfunction in schizophrenia may be present already at the early stages of auditory processing of simple stimuli, and not only at stages involving higher-order semantic processing. This dysfunction involves an impaired lateralization of cortical activation in the secondary auditory cortex (planum temporale). Synchronized activity as measured with MEG was more affected than the metabolic changes measured by fMRI. Potential volume differences in the superior temporal gyrus between patient and comparison groups in our study were corrected by the fMRI analysis method. In line with our findings are results from studies using structural MRI. They found a reduction in the volume of the left planum temporale (secondary auditory cortex) but not Heschl’s gyrus (primary auditory cortex) in patients
(67–
69) (for review see references
70,
71). We therefore could provide a potential link between altered planum temporale morphology in schizophrenia and its functional consequences, i.e., disrupted preattentive acoustic processing.
In conclusion, we found altered neural responses to basic sound processing at the level of the planum temporale in a group of patients with schizophrenia in line with morphological changes in this region. Although the underlying pathology remains elusive, it might be related to deficient NMDA-dependent neurotransmission
(3) and impaired synaptic connectivity
(72) and thus reduced stimulus-induced phase locking of cortically evoked activity. The superior temporal gyrus is a crucial area in the generation of language-related symptoms in schizophrenia such as formal thought disorder
(24) and auditory hallucinations
(23), so this region warrants attention in future research.