Smooth pursuit eye movement abnormalities are among the most reproducible physiologic abnormalities associated with schizophrenia
(1). Tracking of a moving target can be performed by two neuronal systems: 1) a smooth pursuit system that precisely matches the foveal area of the retina to the visual image of the target or 2) saccadic movements that rapidly bring the target image to the fovea. Normal subjects perform smooth pursuit eye movement tasks using their smooth pursuit system almost exclusively. Many investigators have proposed that the fundamental deficit in schizophrenia involves a failure to inhibit the saccade system, resulting in the intrusion of saccades during the smooth pursuit task
(2–
4). Small saccades ahead of the target motion that span less than 4° of the visual field (leading saccades) show the most significant differences in schizophrenia
(5). Other researchers have emphasized the importance of low pursuit gain (the relationship of the velocity of the eye movement to the velocity of the target) and the concomitant increase in compensatory (catch-up) saccades
(6–
8).
Most studies investigating the functional neuroanatomy of smooth pursuit abnormalities have tested hypotheses indirectly by exploring the relationship between pursuit quality and performance on tasks thought to isolate distinct components of the pursuit task. For example, Chen and colleagues
(9) reported that patients with schizophrenia have a diminished ability to discriminate the velocity of moving targets, arguing for the involvement of primary motion processing areas of the brain such as the middle temporal visual area (V5) and the middle superior temporal area. Examination of pursuit initiation and maintenance with foveofugal and foveopetal ramp tracking tasks has demonstrated the importance of higher visual centers such as the frontal eye fields and their cortical connections in smooth pursuit abnormalities
(8). Pursuit-related frontal eye field dysfunction has also been observed in a PET study examining relatives of schizophrenic patients
(10). Altered resting frontostriatal glucose metabolism has also been associated with pursuit abnormalities in schizophrenia
(11).
To our knowledge, no study has used functional magnetic resonance imaging (fMRI) to investigate the neurobiology underlying smooth pursuit abnormalities in patients with schizophrenia. Compared to other brain imaging techniques, fMRI offers improved spatial and temporal resolution for studying physiologically complex tasks. This study examines differences in brain hemodynamic response between healthy comparison subjects and patients with schizophrenia during performance of a smooth pursuit eye movement task.
Method
Subjects
Fourteen healthy comparison subjects and 14 subjects with DSM-IV schizophrenia participated in this study. Comparison subjects included five men and nine women, with a mean age of 37.6 years (SD=10.6). Subjects with schizophrenia also included five men and nine women, with a mean age of 39.9 years (SD=7.6). No significant difference in age was found between the groups (t=0.51, df=24, p=0.61). Two additional comparison subjects and four additional subjects with schizophrenia were excluded because of excess head motion (>1 mm) during scanning. Two of the 14 subjects with schizophrenia were neuroleptic free. The subjects refrained from smoking for at least 30 minutes before the study. All participants provided written, informed consent according to a procedure approved by the University of Colorado Institutional Review Board.
Task Design
fMR images were obtained while subjects performed a smooth pursuit task
(12). The task consisted of visually tracking a small white dot that moved horizontally back and forth over a visual angle of 26° at a constant velocity of 16.7° per second followed by a 700-msec fixation period at the edges. For each of four runs in this blocked design, a 10-second equilibration period was followed by four cycles of 25-second task/25-second rest. During the rest period, the subjects were instructed to look straight ahead at the blank screen.
MR Parameters and Data Analysis
Studies were performed with a 1.5-T Siemens Vision MR system (Siemens AG, Iselin, N.J.) by using a standard quadrature head coil. At the beginning of each study, a high-resolution three-dimensional T1-weighted anatomical scan was acquired (TR=45, TE=20, flip angle=45°, matrix=256×256, field of view=240×240 mm, 1.5-mm thick coronal slices). Functional images were acquired by using gradient-echo echo-planar imaging (TR=2500, TE=50, matrix=64×64, field of view=240×240 mm). Each volume included 20 6-mm thick axial slices angled parallel to the planum sphenoidale. There was a 1-mm gap between slices. Eighty volumes were collected for each of four runs in the study over a 20-minute period.
Quantitative Analyses
Spatial preprocessing, model specification and estimation, and statistical inference were performed with SPM 99
(13). The four runs from each subject were concatenated. Images were motion corrected, normalized to the Montreal Neurological Institute template, and subsequently converted to Talairach space
(14). A 4-mm full width at half maximum Gaussian smoothing kernel was then applied. After reslicing for motion correction, normalization to stereotactic space, and application of the smoothing kernel, the effective smoothing was approximately 8×8×8 mm
3. The model consisted of a simple boxcar function (task versus rest), convolved with a hemodynamic response function.
To account for both between-group and between-subject variation, a random-effects analysis was implemented for the whole brain. Parameter estimates for each individual’s analysis (SPM contrast images) were entered into a second-level analysis consisting of a two-sample t test, contrasting for the conditions of comparison subjects > schizophrenia patients and schizophrenia patients > comparison subjects. The second-level model was thresholded at p<0.05 (t=4.72, df=26), corrected for multiple comparisons by using the false discovery rate method
(15).
A supplemental region-of-interest analysis was performed by using the “MarsBar” toolbox in SPM 99
(16). A priori regions consisted of 10-mm-diameter spheres placed in the frontal eye fields, supplementary eye field, parietal eye fields, cingulate gyrus, and occipital region; these areas have previously been shown to be involved in smooth pursuit eye movements in healthy individuals
(17–
19). The mean time course for all voxels in each region was analyzed by using a random-effects analysis as described earlier. Data for each region were thresholded at p<0.05 (t=2.48, df=26), with Bonferroni correction for the number of regions evaluated.
Qualitative Analyses
To assess the variability in activity related to smooth pursuit eye movements in comparison subjects and patients with schizophrenia, a statistical parametric map was created for each subject, as described earlier, and was thresholded at p<0.005, corrected (false discovery rate). Regions previously identified as being involved in smooth pursuit eye movements and regions showing different levels of activation between the groups in this study were evaluated qualitatively. A region was considered to contain “activity” if any above-threshold voxels were present. A chi-square test was performed to determine the significance of between-group differences in activity in those regions.
Head Movement and Voxel-Based Morphometric Analyses
To test for differences in head movement between the groups, realignment parameters from the SPM 99 realignment routine were compared. Movement information included magnitude of movement from the original position in three translational (x-, y-, and z-axes) and three rotational (roll, pitch, and yaw) directions for each of the 320 time points acquired for each subject. No significant between-group differences were found.
A voxel-based morphometric analysis was employed to determine if differences in activation were due to gross differences in anatomy between the groups
(20). Group differences were evaluated by using the general linear model in SPM 99
(13), thresholded at p<0.01, and corrected for family-wise error (t=5.91, df=26).
Infrared Oculography
Subjects’ performance on the smooth pursuit eye movement task was assessed outside the magnet with infrared oculography
(4) to detect eye movements such as anticipatory saccades and to calculate pursuit gain. Anticipatory saccades are jumps in the direction of target motion, beginning and ending ahead of target location or increasing position error by 100%, followed by a 50-msec interval of eye velocity less than 50% of target velocity. All segments of pursuit were included in the gain calculation so that gain was retained as a global measure of task performance
(12). Age-adjusted z scores were computed for each individual
(4). The pursuit task was identical to that used in the magnet.
In-Magnet Eye Movement Analysis
To assess compliance with the task while subjects were in the scanner, a power spectrum analysis of the time series data was performed to examine task-correlated fluctuations in voxel intensity in the region of the optic nerve. Time series data were obtained from SPM normalized echo-planar images. Data were averaged from an 8×8×1 voxel region of interest placed over the eye and optic nerve. In order to remove a large zero-order power component and reduce spurious high-frequency power, the mean was subtracted, and a Hamming filter was applied to taper the ends of the data. The spectrum was calculated by using the square of the magnitude of the Fast Fourier Transform. In addition, spectra were normalized by dividing spectrum values by the total power. On the basis of the task paradigm and volume acquisition time, the expected intensity fluctuations caused by eye movement were between 0.16 and 0.2 Hz. Accordingly, the area under the curve of this portion of the spectrum was calculated, and results were compared between fixation and tracking states, as well as between groups
(21).
Results
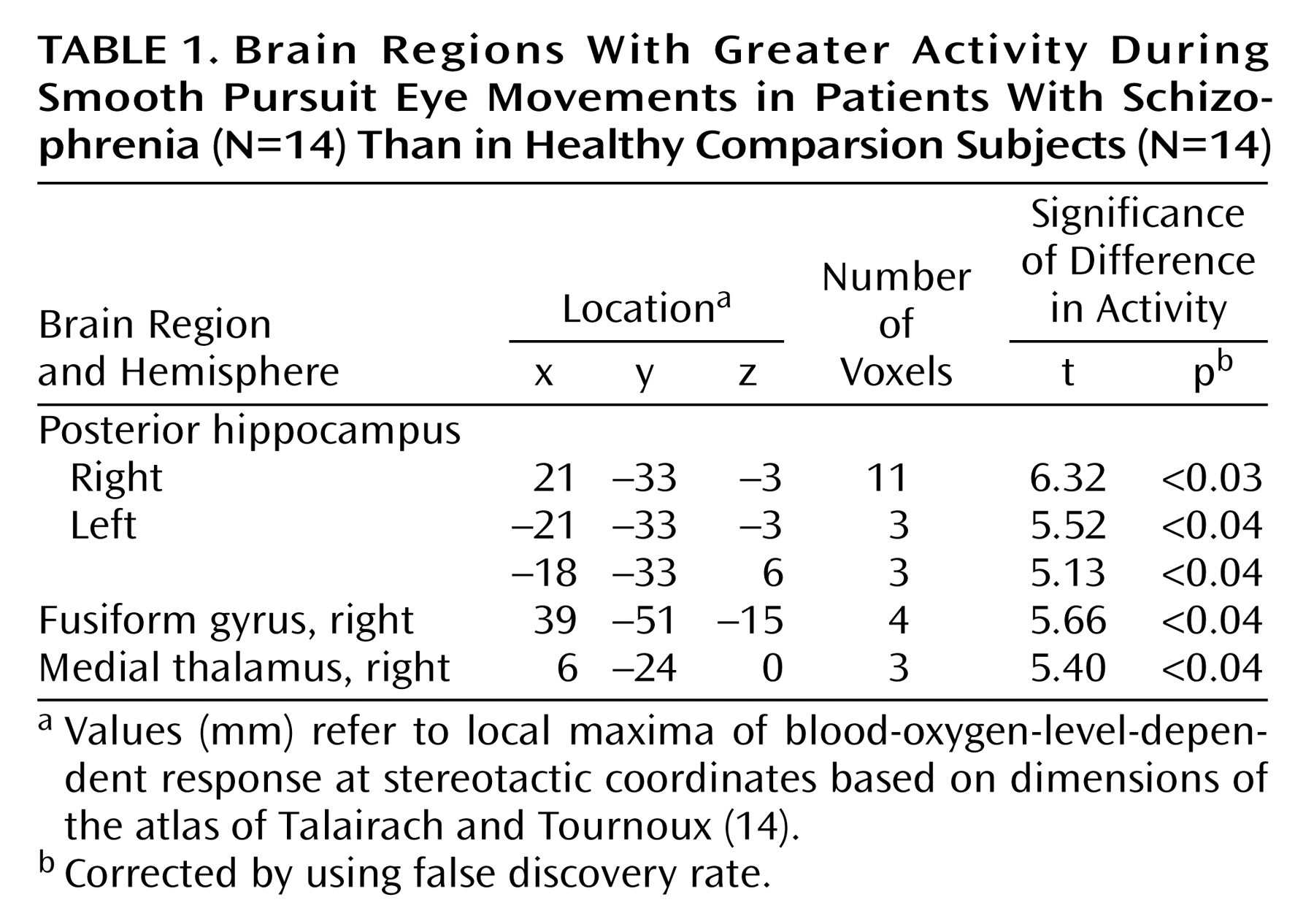
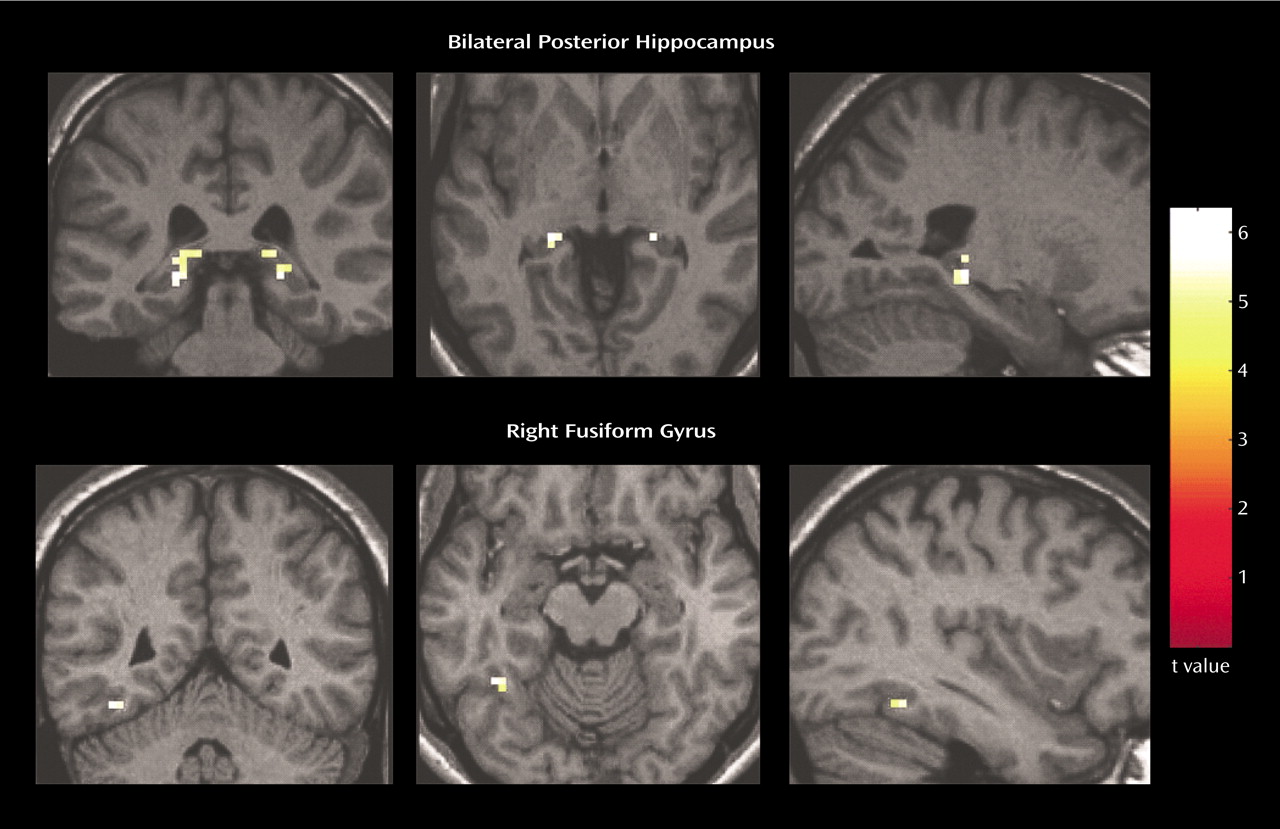
Relative to the comparison subjects, the patients with schizophrenia exhibited greater activity associated with a smooth pursuit eye movement task in multiple brain regions. The patients with schizophrenia demonstrated significantly (p<0.05, corrected) greater activity in the posterior hippocampus bilaterally and in the right fusiform gyrus (
Figure 1). A small area of greater activity was also observed in the medial thalamus (
Table 1).
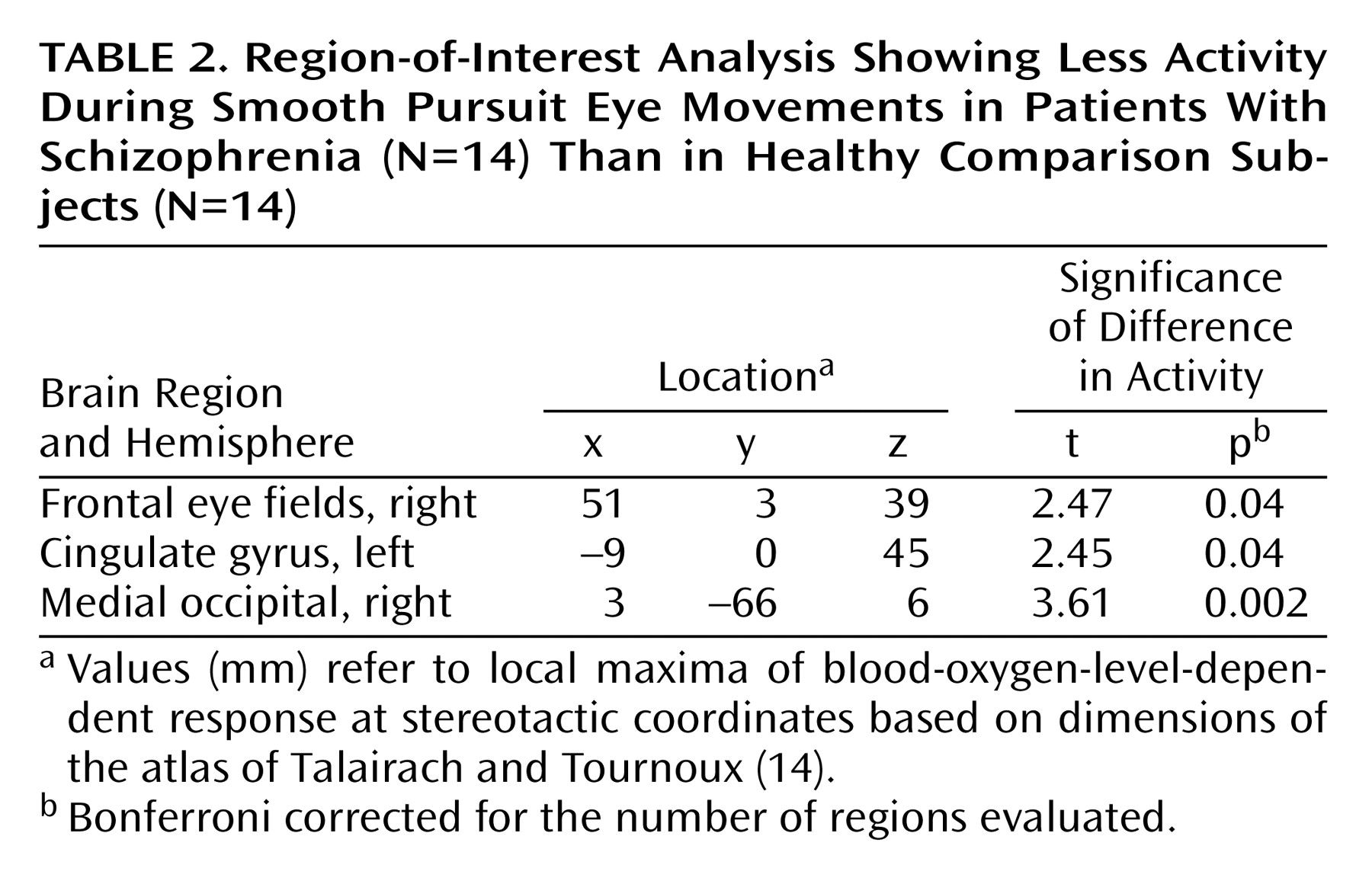
No areas of significantly lower activity in the patients with schizophrenia, relative to the comparison subjects, during the pursuit task were observed by using the whole-brain analysis method in this study. Lower activity in the schizophrenia subjects was observed in the frontal eye fields, occipital region, cingulate gyrus, and superior temporal gyrus, but differences in these areas did not reach significance in the stringent random-effects analysis used for the whole-brain analysis.
The region-of-interest analysis examining brain areas known a priori to be involved in smooth pursuit eye movements in healthy individuals revealed lower activity in patients with schizophrenia relative to the comparison subjects in the right frontal eye field, cingulate gyrus, and occipital region (
Table 2).
Qualitatively, all comparison subjects and schizophrenia subjects showed pursuit-related activity in the frontal eye fields, parietal eye fields, area V1 and middle temporal visual area/middle superior temporal area, and cerebellum. Most, but not all, subjects demonstrated activation in the supplementary eye field (12 of 14 comparison subjects and 10 of 14 schizophrenia subjects), cingulate gyrus (14 of 14 comparison subjects and 11 of 14 schizophrenia subjects), and thalamus (10 of 14 comparison subjects and 11 of 14 schizophrenia subjects). In contrast, significantly more patients with schizophrenia, relative to the comparison subjects, showed activity in the hippocampus (three of 14 comparison subjects, compared with 11 of 14 schizophrenia subjects, p<0.01, Fisher’s exact test) and fusiform gyrus (four of 14 comparison subjects, compared with 12 of 14 schizophrenia subjects, p<0.01, Fisher’s exact test).
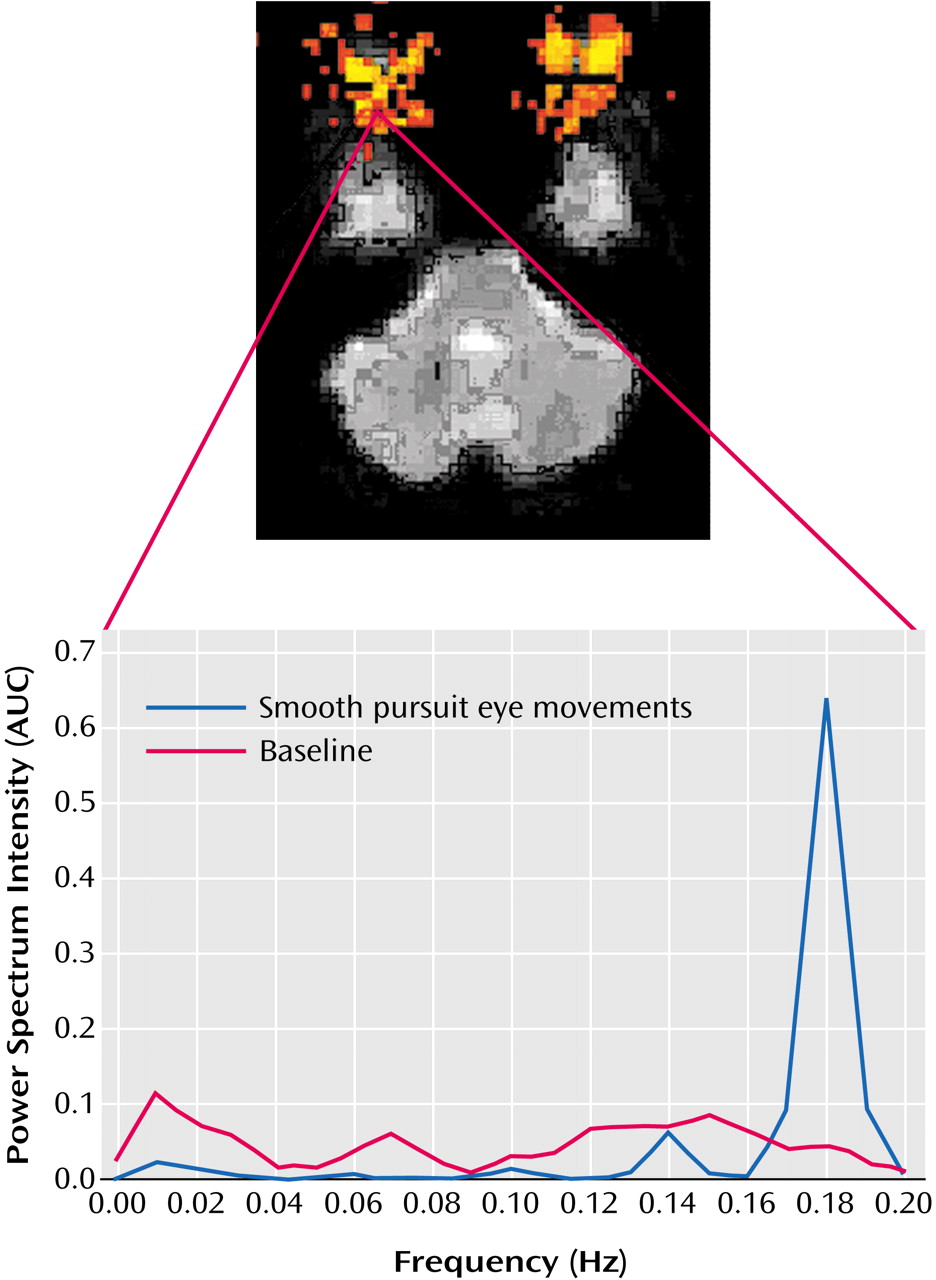
To assess subjects’ eye movements in the scanner, Fourier analyses of echo-planar image time series data from a region of interest covering the eye were performed. This technique proved sensitive to eye movements at the expected frequency of 0.2 Hz (
Figure 2). No significant difference between groups in eye movements during the rest (baseline) condition was observed (comparison subjects: mean=289, SD=81.9; schizophrenia subjects: mean=293, SD=66.9) (t=0.14, df=26, p=0.89). A significant group difference was observed during performance of the smooth pursuit task (comparison subjects: mean=896, SD=224; schizophrenia subjects: mean=696, SD=243) (t=2.27, df=26, p<0.03).
The voxel-based morphometric analysis did not reveal any between-group differences in gray matter in regions that showed greater pursuit-related activation in the patients with schizophrenia. Significant (p<0.01, corrected) decreases in the concentration of gray matter in subjects with schizophrenia were observed in the inferior frontal cortex and a small portion of the dorsolateral prefrontal cortex.
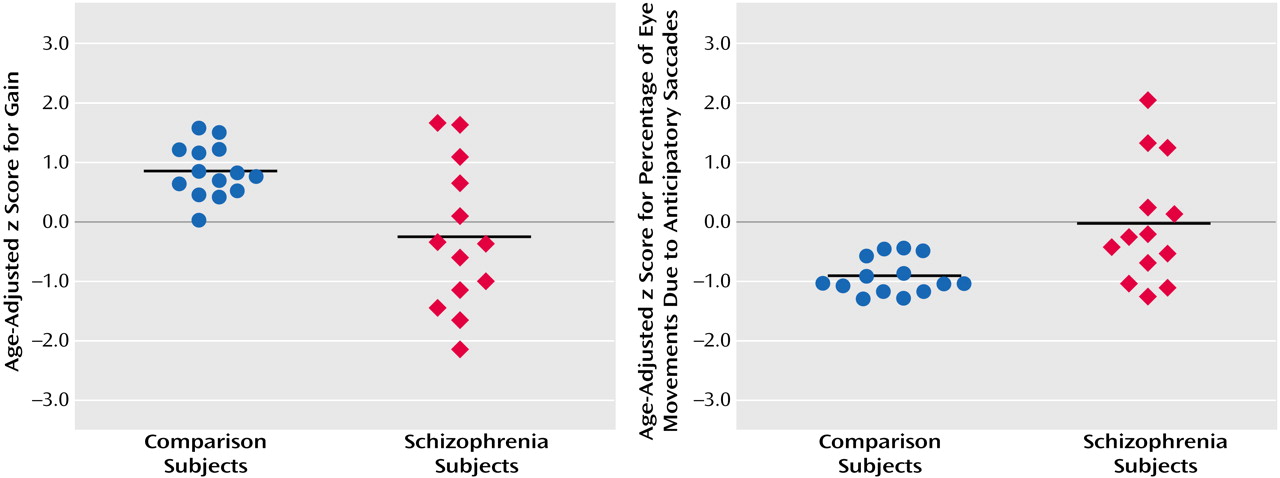
Comparison subjects had a mean age-adjusted z score for gain (eye velocity relative to target velocity) of 0.82 (SD=0.44) and a mean age-adjusted z score for percentage of total eye movements due to anticipatory saccades of –0.94 (SD=0.30) (
Figure 3). Subjects with schizophrenia had a mean age-adjusted z score for gain of –0.27 (SD=1.24) and a mean age-adjusted z score for percentage of anticipatory saccade of –0.03 (SD=1.02). Eye tracking data were not available for one subject who could not be reached. Both measures of smooth pursuit function were significantly different between the groups (gain: t=3.0, df=15, p<0.009; percentage of anticipatory saccades: t=3.1, df=14, p<0.008).
As an additional exploratory analysis, fMRI data from the schizophrenia patients with the poorest gain (N=6) were compared with data from the patients with the highest gain (N=6) by using a second-level random-effects model as described earlier, thresholded at p<0.005 (t=3.17, df=10). Compared to the group with low gain, the group with high gain showed significantly greater activation in the middle temporal visual area (bilateral) and smaller areas of greater activation in the left insula, left superior frontal gyrus, anterior cingulate, and superior temporal gyrus. Patients with poor gain demonstrated relatively greater activation in the cerebellum. Smaller areas of greater activation in poor trackers were observed in the middle frontal gyrus, inferior frontal gyrus, and posterior cingulate.
A comparison was also made between the comparison subjects and subgroups of patients whose pursuit performance was either similar to or poorer than that of the comparison subjects. Patients whose performance was similar to that of the comparison subjects (N=5) showed robust elevations in posterior hippocampus activity (t=3.36, df=8, p<0.005), relative to the comparison subjects. Smaller elevations in activity were observed in the posterior hippocampi, lateral geniculate nuclei, posterior thalamus, and posterior cerebellum in patients who performed poorly, relative to the comparison subjects (N=7).
Discussion
The major finding of this study is greater pursuit-related activity in the posterior hippocampus and right fusiform gyrus in patients with schizophrenia, relative to healthy comparison subjects. In addition, a region-of-interest analysis of areas known to be involved in smooth pursuit revealed differences in frontal areas, including lower activation in the frontal eye fields, cingulate gyrus, and occipital region.
The involvement of the hippocampus and fusiform gyrus in eye movement tasks has been demonstrated with single-unit recordings in primates
(22,
23) and with PET studies in humans
(24,
25). Differential activation of these regions during a smooth pursuit task in schizophrenia is consistent with neuropathological and neurophysiological evidence for inhibitory dysfunction of these regions, particularly the hippocampus, in the disease
(26,
27). The finding of greater hippocampal activation in patients with schizophrenia during a smooth pursuit eye movement task in this study suggests that abnormalities in their eye movements may also involve failure of inhibition in the hippocampus. Greater hippocampal activation in schizophrenia patients whose pursuit performance was either poorer than or similar to that of the comparison subjects suggests that hyperactivation of this region may not be associated with pursuit quality but rather with a diagnosis of schizophrenia.
Our findings also suggest involvement of other brain regions in smooth pursuit dysfunction in schizophrenia. Region-of-interest analysis of smooth pursuit-related areas revealed differences in frontal function in the schizophrenia patients. Specifically, the lower activation of frontal eye field observed in this study is consistent with previous PET studies demonstrating lower pursuit-associated activity in the frontal eye fields in relatives of patients with schizophrenia
(10) and lower resting frontal eye field glucose metabolism in schizophrenia
(10,
11,
28). Frontal eye field dysfunction may be particularly important in the low gain component of pursuit dysfunction in schizophrenia
(8). Frontal regions such as the frontal eye fields and cingulate gyrus may also play a role in the saccadic intrusion component of smooth pursuit abnormalities
(29). In a recent study by Rosano and colleagues
(30), high-resolution imaging at 3 T was used to distinguish subregions of the frontal eye fields that were related to smooth pursuit from subregions related to saccades. While the resolution used in the present study limits our discussion to the larger functional area, the interpretation of lower activity in this region contributing to greater saccadic intrusion is consistent with a recent human neurosurgical mapping study by Milea and colleagues
(31), in which the investigators stimulated the frontal eye fields with electrodes. Frontal eye field stimulation elicited spontaneous contraversive smooth pursuit and interfered with saccadic eye movements. It seems reasonable to speculate, therefore, that a gross deficit in activity in this region may lead to lower quality of smooth pursuit caused by a greater number of intrusive saccades.
In addition to the involvement of frontal areas in pursuit dysfunction, this study demonstrated the involvement of posterior visual processing areas such as the fusiform gyrus. This region is known to be involved in higher visual processing, particularly for faces and other shape recognition
(32). While the fusiform has received a great deal of attention for its role in the processing of faces, and it can be functionally divided into anterior and posterior regions based on this function, less is known about its role in processing other visual information. The region shown to be differentially activated in this study has been shown to be active in healthy comparison subjects during passive viewing of a field of many horizontally moving stimuli (square blocks, very similar to the smooth pursuit stimulus used in the present study), compared to fixation
(33), suggesting that this subregion of the fusiform gyrus may play a role in processing motion information. Malaspina and colleagues
(34) demonstrated increased cerebral blood flow in the right fusiform during visual fixation in schizophrenia.
Primary sensory and motor areas may also be involved in pursuit dysfunction in schizophrenia. In the within-group analysis comparing schizophrenia patients with high versus low gain, the middle temporal visual area, which is known to be involved in motion processing, was significantly more active in the good trackers. This finding may be consistent with the hypothesis of impaired velocity discrimination in schizophrenia
(9). In addition, the posterior cerebellum was more active in the poor trackers, relative to both the good trackers with schizophrenia and to the comparison subjects. This region plays a role in coordinating saccadic and smooth pursuit eye movement signals from the frontal cortex, and it has been suggested that this region is involved in pursuit dysfunction in schizophrenia
(35).
One limitation of this study is that we are not able to exclude the possibility that observed differences may be related to differences in regional cerebral blood flow (rCBF) between the groups
(36–
38). It is also difficult to exclude neuroleptic effects. It has been shown, however, that typical neuroleptics do not alter smooth pursuit performance
(39) or rCBF in patients with schizophrenia
(38). Smooth pursuit-associated brain activation patterns in the two neuroleptic-free patients in this study were qualitatively similar to those observed in the medicated patients.
Finally, it is possible that differences in brain activation may have been due to differential performance in the baseline rest condition, in which all subjects were instructed to “look straight ahead” at the black screen. This explanation seems unlikely, however, as the Fourier analysis of eye movement detected in the echo-planar images revealed no significant difference in baseline performance. In addition, to test for the possibility of group differences in brain activation during rest, we explicitly contrasted for both higher and lower levels of activity between groups during rest and found no differences (data not shown).
In conclusion, this study has identified temporal and frontal lobe differences in brain activity associated with a smooth pursuit eye movement task in schizophrenia. The largest observed difference was a relatively higher level of activity in the bilateral posterior hippocampus in the subjects with schizophrenia. This observation is consistent with the hypothesis that inhibitory interneurons in the hippocampus are dysfunctional in schizophrenia. Differences in the fusiform gyrus and frontal regions were also observed. The relatively hyperactive temporal and hypoactive frontal regions observed in this study are consistent with the hypothesis of abnormal functional connectivity between these two brain areas in schizophrenia
(40).