Postmortem and functional and structural imaging studies have supported this hypothesis, with structural magnetic resonance imaging (MRI) studies suggesting that several of the heteromodal association cortex components are characterized by gross structural changes. In particular, there is general consensus that total prefrontal gray matter volume is smaller in patients with schizophrenia, relative to healthy comparison subjects
(3). However, there is little agreement about whether this smaller volume represents a global deficit or is restricted to the prefrontal subcomponents. Studies that have examined specific prefrontal regions
(4,
5) or have parcellated the prefrontal cortex into subcomponents
(6–
12) have variously observed smaller volumes of the dorsolateral, superior/medial, inferior, or orbital prefrontal regions in patients with schizophrenia. The lack of consensus among studies is due, in large part, to variability in the number and definitions of the prefrontal regions.
The very few studies that have concurrently examined more than one heteromodal association cortex region
(4,
5,
16) have examined whether the region’s potential selective involvement in the neuroanatomy of schizophrenia results in unique relationships among these structures. Previous studies of the relationships among heteromodal association cortex regions have produced divergent results. In a factor analytic study, measures of the dorsolateral prefrontal cortex and superior temporal gyrus loaded onto the heteromodal association cortex factor in both patients with schizophrenia and healthy comparison subjects
(17). The inferior parietal cortex did not contribute to this factor in either group. In a second study, frontal lobe and superior temporal gyrus volumes were significantly more correlated with each other in healthy comparison subjects than in subjects with schizophrenia
(5). In contrast, Niznikiewicz and colleagues
(16) observed that volumes of the right and left inferior parietal cortices were significantly correlated with volumes of the right and left inferior and orbital, left middle, and right superior prefrontal regions in patients with schizophrenia but not in healthy comparison subjects. In patients with schizophrenia, volumes of both the right and the left inferior parietal regions were significantly correlated with the left anterior superior temporal gyrus volume
(16).
We previously reported the development of a reliable procedure for parcellating the prefrontal cortex into four subcomponents and found that patients with schizophrenia had selective smaller volumes of right and left inferior prefrontal region gray matter
(6). The procedure is based on the method proposed by Rademacher and colleagues
(18) and utilizes surface sulcal landmarks, information on the functional organization of the brain, and three-dimensional MRI assessment methods. The current study was designed to replicate our previous observation of smaller inferior prefrontal region volumes, extend the evaluation of heteromodal regions to the superior temporal gyrus and inferior parietal cortex, and examine the pattern of correlations among the heteromodal regions. We hypothesized that patients with schizophrenia would be characterized by smaller heteromodal cortical volumes, relative to healthy comparison subjects, and that the pattern of volumetric correlations among the heteromodal regions would differ between the two groups.
Method
Subjects
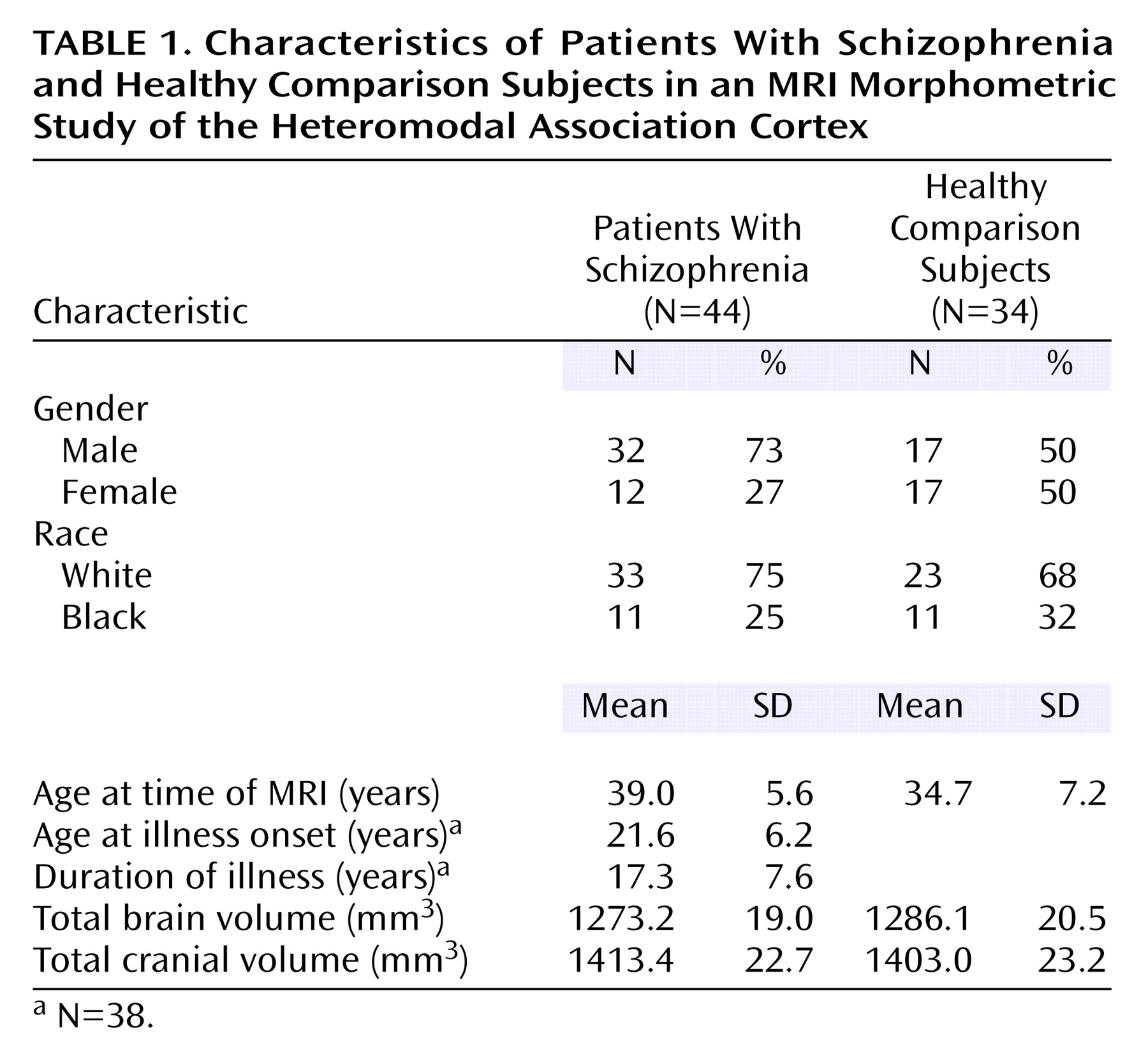
Forty-four outpatients with DSM-III-R/DSM-IV schizophrenia or schizoaffective disorder were selected for study entry. Patients’ diagnoses were based on the Structured Clinical Interview for DSM-III-R/DSM-IV (SCID)
(19,
20), interviews with family informants, and past medical records. Thirty-four healthy comparison subjects were recruited from a group of community volunteers. Comparison subjects were excluded if they had a history of a DSM-III-R/DSM-IV axis I or axis II disorder on the basis of a SCID interview. Patients and comparison subjects were excluded if they had a history of neurological disorder, mental retardation, head injury with loss of consciousness for greater than 30 minutes, or a diagnosis of substance abuse or dependence within the last 12 months. Forty-one patients and 33 comparison subjects were right-handed. Fifteen patients were treated with conventional antipsychotics, seven patients were treated with new-generation antipsychotics other than clozapine, and 16 patients were treated with clozapine. Medication data were missing for six patients. All subjects provided written informed consent before study entry.
MRI Protocol
MRI studies were performed on a 1.5-T Signa GE Scanner (General Electric, Milwaukee). The whole brain was evaluated in the coronal plane by using a spoiled gradient recall acquisition in the steady-state three-dimensional imaging sequence, with the following imaging parameters: TR=35 msec, TE=5 msec, flip angle=45°, number of excitations=1, field of view=24 cm, matrix size=256 ×256, and 1.5-mm slice thickness.
Image Processing and Measurement
The MEASURE system was used to process and analyze images
(6,
21). Individual coronal MRI images are stripped of all nonbrain tissue and combined to form a three-dimensional representation of the brain. The three-dimensional representation and the three MRI image orthogonal views (coronal, axial, and sagittal) are displayed on the computer screen. The surface sulcal landmarks for each region are identified and painted on the three-dimensional representation of the brain (see figure 1 in reference
6). The demarcated regions are then painted with different colors, with each paint color defining the set of voxels used to calculate cortical region volume. The paint colors are depicted on the orthogonal views. The painted orthogonal views and three-dimensional brain representation are used in an iterative manner to identify the voxels of each brain region. A stereological method is used to count the gray matter and white matter voxels
(21,
22). In this method, a three-dimensional grid of predetermined size is superimposed on the MRI images; the individual grid points were represented by small rotated “L” shapes (see figure 3 in reference
6). Optimal grid size is based on the Cavalieri principle and was calculated by using the formula developed by Gunderson et al.
(22). Gray matter volume is calculated by counting the number of L’s that contain a gray matter voxel along the diagonal defined by the angle of the L; white matter volume is calculated by counting the number of remaining L’s within the region of interest
(22).
Anatomical Boundaries
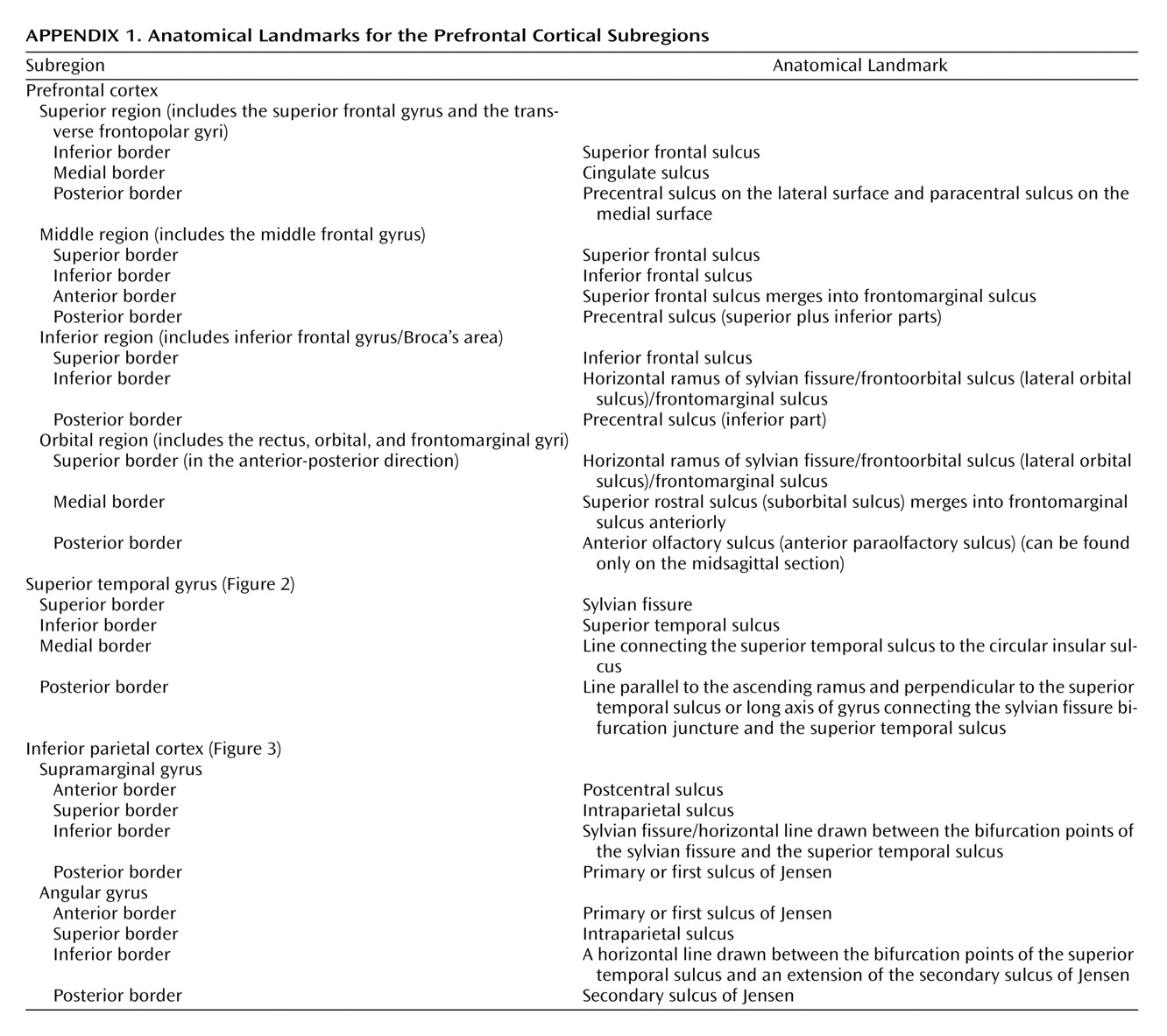
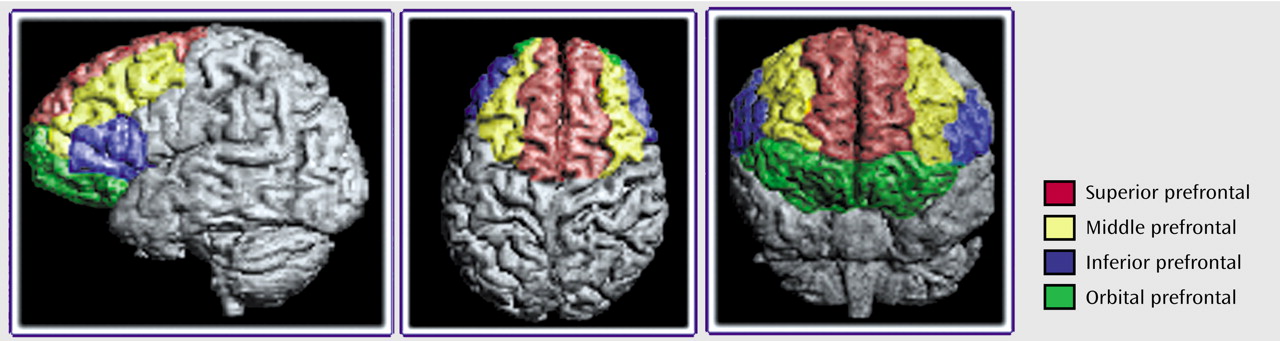
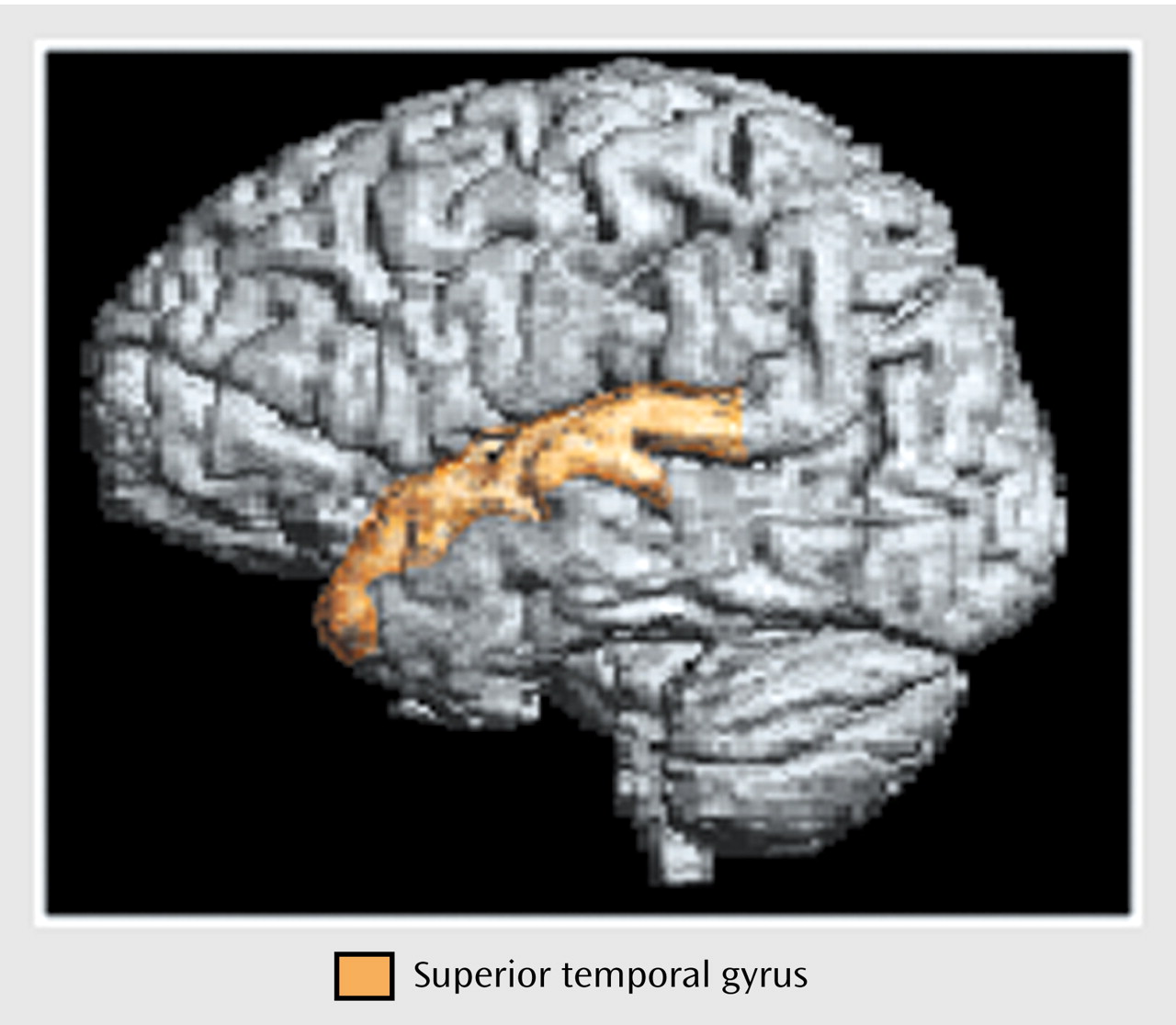
Anatomical boundaries for each region are described in
Appendix 1 and illustrated in
Figure 1,
Figure 2, and
Figure 3. The boundaries are based on surface sulcal landmarks
(23). Prefrontal total gray matter volume is the sum of the four prefrontal region gray matter volumes. Prefrontal total white matter comprises the white matter on each coronal slice identified by the surface paint. If gyri represented on a coronal slice are not part of the prefrontal cortex, then the white matter, defined by the cortical strip and a straight line that connects the apex of each sulcus used to delineate this region from a prefrontal region, is not included in the prefrontal total white matter volume measurement
(6). Superior temporal gyrus white matter volume includes all white matter between the cortical strip and the medial border of the region. The inferior parietal total gray matter volume is the sum of the supramarginal and angular gyri volumes. Inferior parietal white matter includes all white matter within the cortical strip and the borders of each gyrus.
Total cranial volume includes the cerebrum, cerebellum, sulcal and ventricular CSF, and brainstem. The inferior boundary of the brainstem is defined by a straight line from the posterior margin of the foramen magnum (most inferior and anterior point in the occipital bone) and the anterior margin of the foramen magnum (most posterior and inferior of the basiocciput). Total brain volume includes all brain structures that define the total cranial volume but excludes the sulcal and ventricular CSF.
Interrater reliability for the prefrontal region, superior temporal gyrus, and inferior parietal region was based on the independent measurement of each region in five brains. The intraclass correlation coefficients were 0.95 for the superior prefrontal region, 0.93 for the middle prefrontal region, 0.91 for the inferior prefrontal region, 0.93 for the orbital prefrontal region, 0.89 for the superior temporal gyrus, 0.93 for the supramarginal gyrus, 0.86 for the angular gyrus, 0.99 for the total brain volume, and 0.99 for the total cranial volume.
Statistical Analyses
To examine effects of diagnosis, gender, and hemisphere, we fitted a mixed analysis of covariance model for repeated measures within each brain region by using the PROC MIXED procedure in SAS Version 8.2 (SAS, Inc., Cary, N.C.), with volumes for the two hemispheres as the repeated measure within subjects. All models were adjusted for age. We used backward hierarchical selection to remove nonsignificant (p>0.05) interaction terms, starting with the following initial model for volume:
volume = age + diagnosis + gender + hemisphere + diagnosis-by-gender + diagnosis-by-hemisphere + gender-by-hemisphere + gender-by-diagnosis-by-hemisphere.
In this procedure, selection began by testing the three-way interaction, dropping that term if it was nonsignificant, then dropping in order the least significant two-way interactions, until only significant interactions and main effects remained. All main effects were retained in the final model. Least squares means and their standard errors were estimated for brain volumes within levels of one factor, averaging over the other terms in the model; where significant interactions were present, least squares means were estimated for all combinations of levels of the factors involved in the interaction. The p values were not adjusted for multiple comparisons. Neither total brain volume nor total cranial volume was included in these models, since there were no significant effects for these measures other than gender differences.
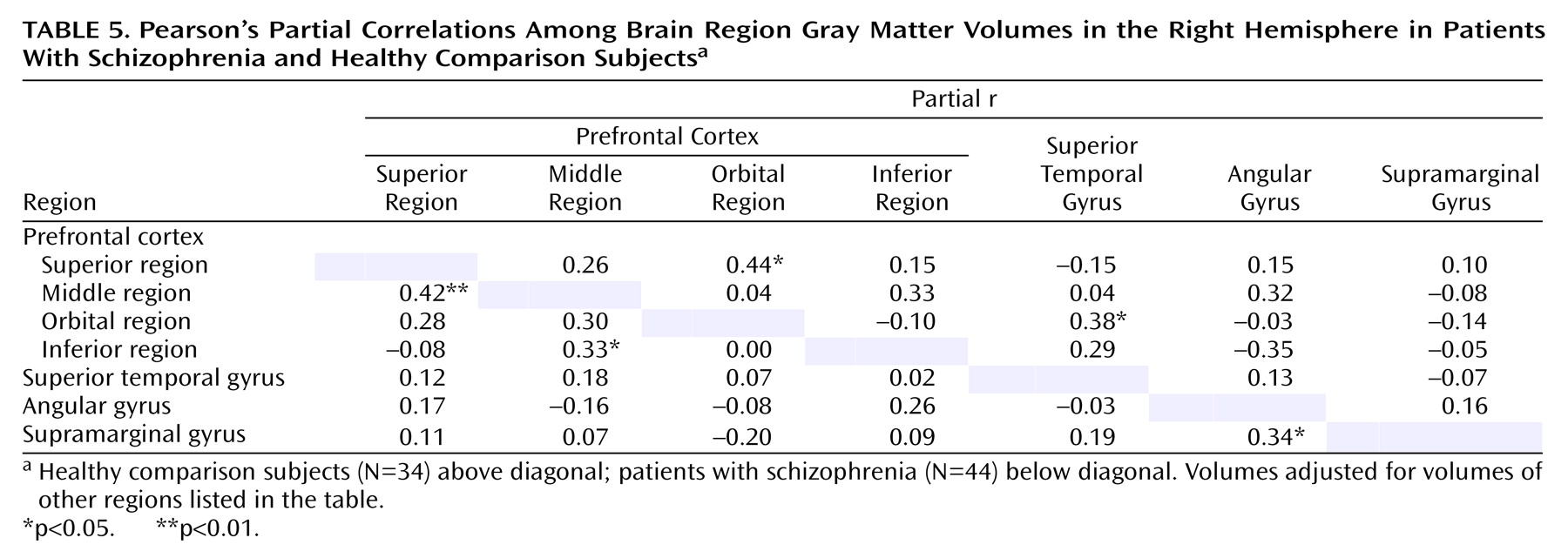
To examine patterns of association among heteromodal regions within each hemisphere, Pearson’s partial correlations were computed between the different gray matter volumes. Each pairwise correlation was adjusted for all other volumes considered, to examine the independent association of the two volumes. These correlations were calculated separately for the comparison subjects and for the patients with schizophrenia. To compare the magnitude of correlations between the two groups, Fisher’s z transformation, z(r)=0.5×log[(1+r)/(1–r)], was applied to each of the compared correlations. The significance of the difference between correlations was then tested by using the asymptotically normal statistic T=(z
patients – z
comparison subjects)/[(1/n
patients – 3)+(1/n
comparison subjects – 3)]
2 (24).
Discussion
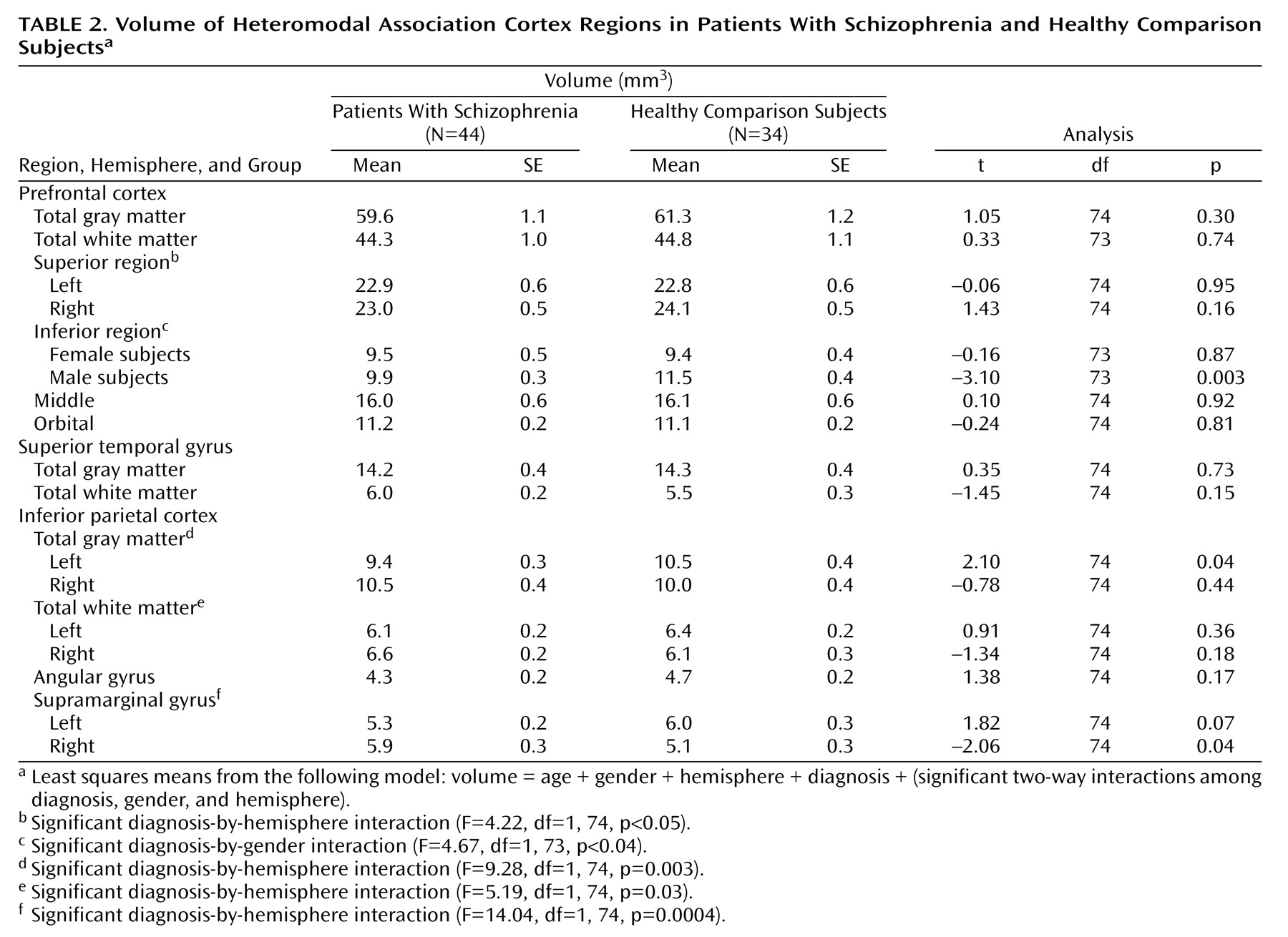
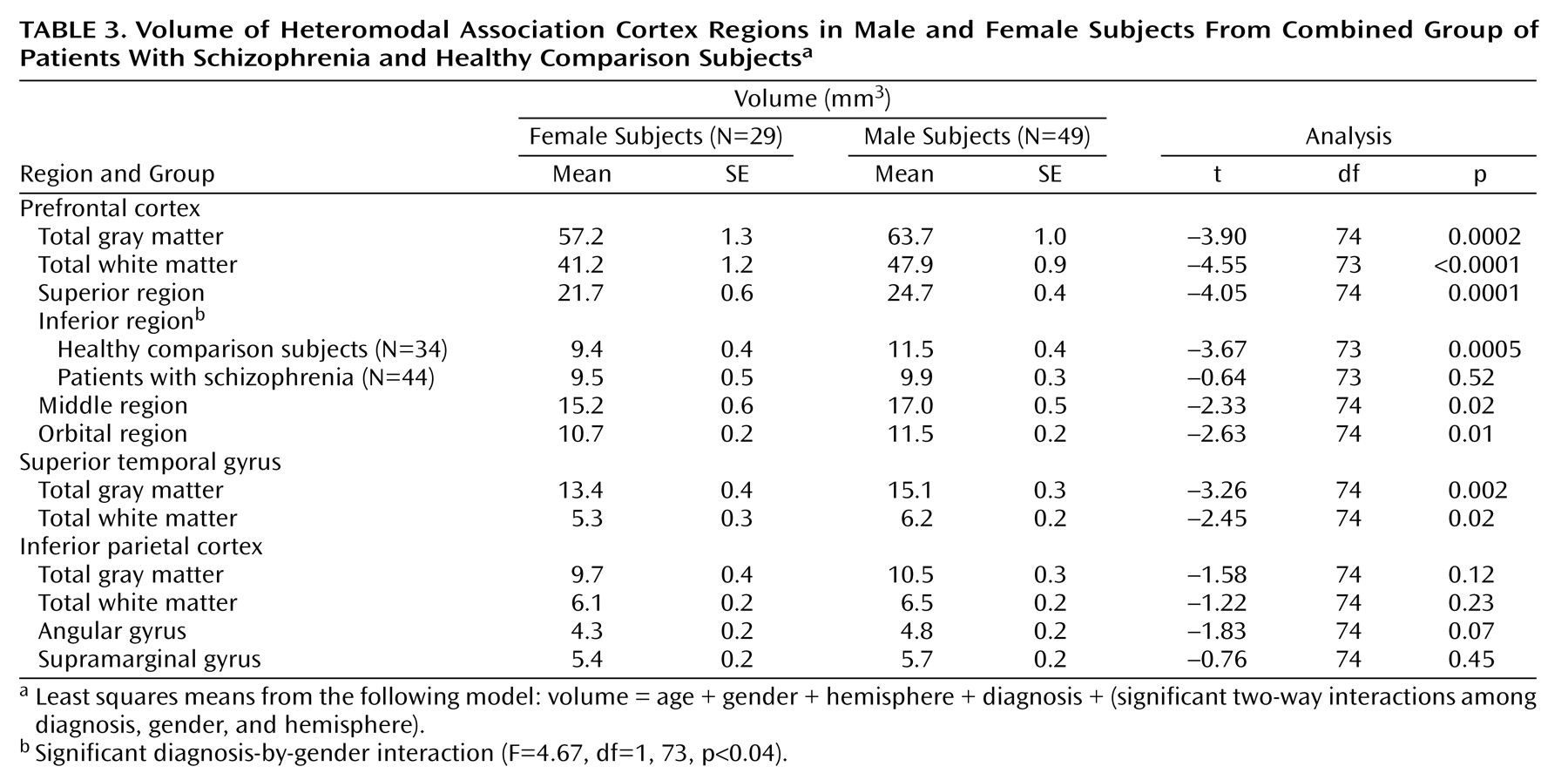
In the current study, patients with schizophrenia were observed to have selective structural abnormalities of the prefrontal and inferior parietal heteromodal cortices, relative to healthy comparison subjects. Male patients exhibited significantly smaller volumes bilaterally in the inferior prefrontal cortex. In our previous study, we also found that volume of this region differentiated the two groups but we did not observe a gender effect because of the small number of female subjects
(6). No other prefrontal region volume differentiated the two groups.
Several previous studies have examined the inferior prefrontal region. Despite marked methodological differences, these studies have shown a consistent demonstration of altered structure in patients with schizophrenia, relative to healthy comparison subjects
(7–
11). Two studies found smaller volumes of this region in male patients with schizophrenia. Wible and colleagues
(7) found smaller volumes in both the left and right inferior frontal gyrus. The magnitude of the group differences was similar to those in the current study: volumes were 10.5% smaller for the left inferior frontal gyrus and 8.3% smaller for the right inferior frontal gyrus in the patients with schizophrenia. In the study by Sanfilipo and colleagues
(11), the inferior lateral region, which would partly correspond to the inferior prefrontal region examined in our study, was bilaterally smaller in male patients with schizophrenia. Neither of these two previous studies included female subjects. In contrast, Crespo-Facorro and colleagues
(9) failed to find left or right inferior prefrontal cortex volumetric differences between first-episode patients with schizophrenia and healthy comparison subjects. Goldstein and colleagues
(8) failed to find a group difference in inferior frontal gyrus volume, but they did not present the data separately by hemisphere. Three studies used voxel-based analytic procedures based on normalized, reformatted images. Two found lower signal intensity in the left inferior prefrontal gyrus in patients with schizophrenia
(25,
26), and one found lower signal intensity in the right inferior prefrontal gyrus in patients with schizophrenia
(26). In a predominantly male group of subjects with schizophrenia, Sigmundsson and colleagues
(27) found an area of lower signal intensity in the left perisylvian region, which included part of the inferior prefrontal region (i.e., Brodmann’s area 44).
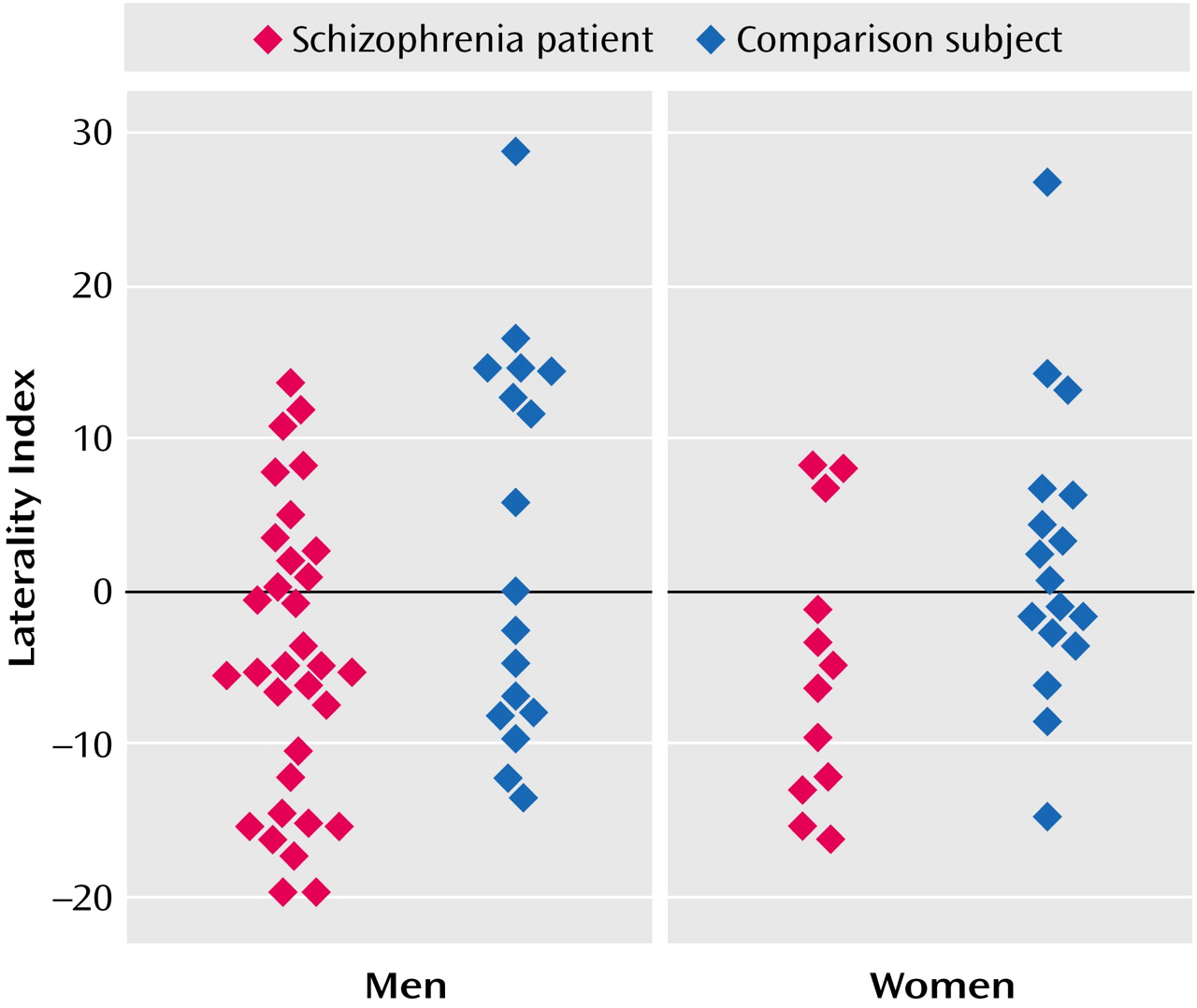
In this study the patients with schizophrenia had a significant reversal of the normal asymmetry of the inferior parietal cortex. The healthy comparison subjects were characterized by larger left inferior parietal total gray and white matter volumes, with the difference due largely to a marked laterality effect for the supramarginal gyrus. The reversal of asymmetry was observed in both male and female patients with schizophrenia. The pattern in the comparison subjects is consistent with previous postmortem and MRI observations of increased size of this region in right-handed subjects in nonpsychiatric populations
(28,
29). In an MRI study of 148 healthy subjects (55% of whom were female), Raz and colleagues
(29) observed a highly significant leftward asymmetry of the inferior parietal cortex and the absence of any gender differences. In contrast, in a group of 30 subjects, Frederikse and colleagues
(30) observed the leftward asymmetry only in male healthy comparison subjects.
Two previous studies have observed reversal of the normal leftward asymmetry of the inferior parietal cortex. Frederikse and colleagues
(15) used an image analytic procedure similar to the one used in the current study. In accordance with the current study findings, they observed that both male and female patients with schizophrenia exhibited a rightward asymmetry of the inferior parietal cortex. However, only the male comparison subjects in their study exhibited the normal leftward asymmetry. The female comparison subjects had rightward asymmetry of this region. The observation of rightward asymmetry of the inferior parietal cortex in the female comparison subjects is in contrast to the current results and to those of Raz and colleagues
(29). In the other study, Niznikiewicz and colleagues
(16) also found a reversal of the normal asymmetry of the inferior parietal cortex in patients with schizophrenia. Their study was limited to male subjects. In the patients with schizophrenia, the rightward asymmetry was the result of hemispheric differences in volume for both the supramarginal and angular gyri, whereas the leftward asymmetry of the healthy comparison subjects was almost completely due to the marked leftward asymmetry of the angular gyrus. In the current study, a similar pattern was seen in the patients with schizophrenia, but the leftward asymmetry of the comparison subjects was due to marked leftward asymmetry of the supramarginal gyrus. Other studies of the inferior parietal cortex have not directly assessed the issue of abnormal inferior parietal cortex asymmetry. Schlaepfer and colleagues
(4) reported smaller volumes of this region in patients with schizophrenia, relative to healthy comparison subjects, with group differences most prominent in female subjects
(4), but hemispheric differences were not described. Goldstein and colleagues
(8) failed to find group differences in inferior parietal cortex total gray matter or angular gyrus volumes, but they did find smaller volumes bilaterally in the posterior portion of the supramarginal gyrus in the patients with schizophrenia.
We found no significant group differences in superior temporal gyrus gray or white matter volumes. These results are in contrast with our previous study, which used the same anatomical landmarks for this region
(31), and with the majority of other studies examining superior temporal gyrus volumes
(3). However, several studies have failed to find a significant group difference for this region
(8,
13,
14). The lack of replication has been attributed to the use of combined gray and white matter superior temporal gyrus measures rather than separate evaluation of superior temporal gyrus gray matter volume
(3). In addition to our study, the study by Goldstein and colleagues
(8) also used a separate measure for superior temporal gyrus gray matter volume and failed to find a group difference. The patient characteristics that might contribute to the variability in study results are unclear.
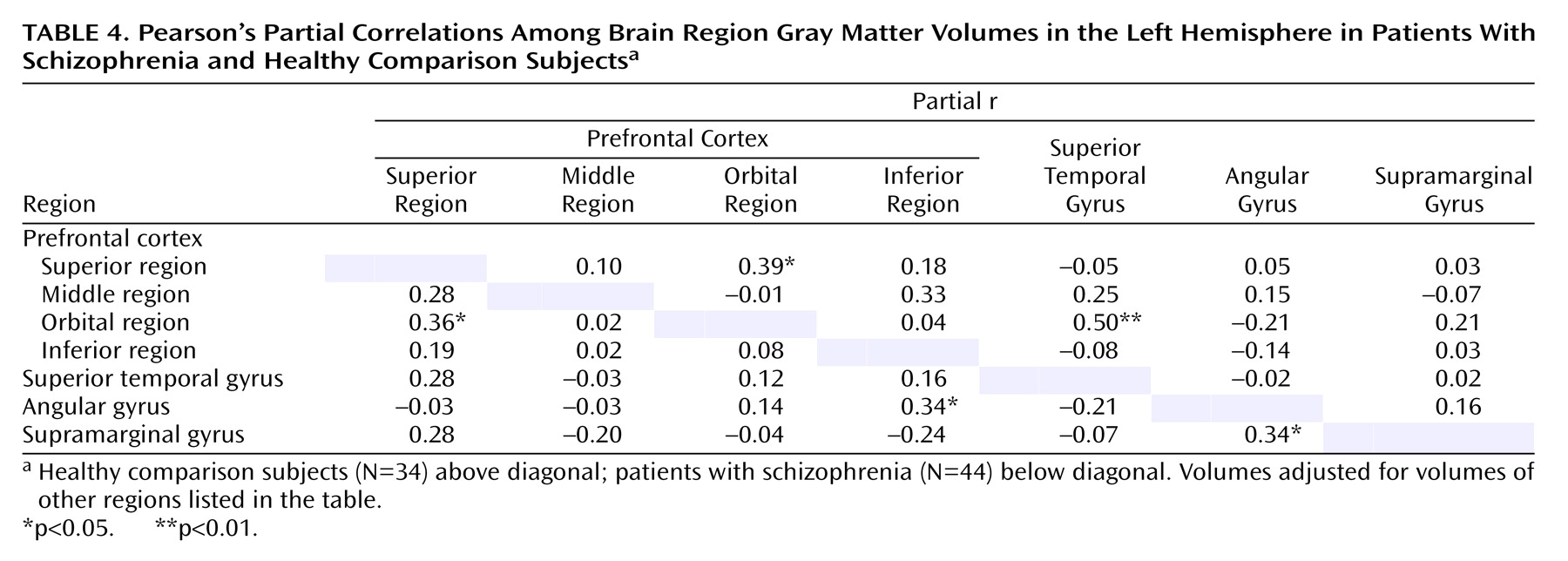
There were few significant correlations of volume among the different heteromodal regions in either the healthy comparison subjects or the patients with schizophrenia. The only significant between-group differences in the correlations were for the correlation between left inferior prefrontal region volume and angular gyrus volume and the correlation between right inferior prefrontal region volume and angular gyrus volume.
Patients with schizophrenia are characterized by a broad range of language and hemispheric lateralization abnormalities
(3,
32,
33). Crow
(34) has hypothesized that these abnormalities may represent the core features of schizophrenia. The current study suggests that these abnormalities may be reflected in structural alterations of the heteromodal cortical areas comprising the language neural circuit
(1). Male patients with schizophrenia had smaller volumes of the left inferior prefrontal region, which consists primarily of Broca’s area. Male and female patients had abnormal asymmetry of the inferior parietal cortex. In right-handed nonpsychiatric subjects, whose language abilities are located in the left hemisphere, the left inferior parietal cortex is larger than the right inferior parietal cortex. Although the precise location of Wernicke’s area is not known, it is thought to include the inferior aspects of the supramarginal and angular gyri
(1). Furthermore, the only correlations for which there were significant group differences were correlations between the inferior prefrontal region and the angular gyrus. In combination, these results suggest that patients with schizophrenia are characterized by abnormal lateralization of the structure and function of the cerebral hemispheres, which leads to abnormalities in language behavior and is reflected in the differential failure of the normal development of the heteromodal language areas.
The current study has several potential limitations. First, the MEASURE image analysis procedure is dependent on the use of reliable rule-based anatomical definitions that conform to local/individual anatomy rather than arbitrary anatomical cut planes. If local neuroanatomy is more variable due to developmental distortions of the usual sulcal gyral patterns in patients with schizophrenia than in healthy comparison subjects
(35), then this variability could lead to differential measurement in the two groups. The high region-of-interest interrater reliability suggests that this potential limitation is not a major concern. Second, the use of the paint method to demarcate the brain regions may be subject to possible inaccuracies, especially in cases of atrophy, where the gray/white and gray/CSF boundaries account for a proportionately greater amount of total gray tissue. Third, the prefrontal cortical regions, other than the inferior prefrontal region, are relatively large and may obscure smaller subregion differences. Fourth, we did not assess the planum temporale, which is part of Wernicke’s area. The failure to measure this region precludes our ability to fully evaluate the heteromodal cortical regions involved in the language neural circuit. Finally, language measures were not available to allow examination of whether the observed structural abnormalities were associated with impairments in language ability.
In summary, we have found evidence for the selective disruption of heteromodal association cortical areas involved in the neuroanatomy of language in patients with schizophrenia. Future studies are needed to delineate the pathophysiological process(es) underlying the structural changes of these brain regions.