Catechol
O-methyltransferase (COMT) is an important enzyme in dopamine metabolism. A common functional polymorphism at codon 158 in the gene coding for COMT (COMT Val
158Met) results in substantial effects, with homozygosity for the low-activity (Met) allele leading to a three- to fourfold reduction in enzymatic activity compared with the high-activity (Val) allele. This polymorphism has been associated with diverse phenotypes in patients with schizophrenia. The Met allele has been associated with aggressive/dangerous behavior (e.g., references
1,
2) but also with better performance on cognitive tasks
(3) and increased prefrontal efficiency
(4). Bilder et al.
(3) further report that symptom ratings were higher in Met homozygotes despite better neurocognitive scores.
These complex associations are difficult to explain by a unitary hypothesis of increased prefrontal dopamine. Manipulations of dopamine can be beneficial or detrimental
(5,
6). The tonic/phasic dopamine theory
(7–
9) may help resolve these apparent discrepancies. Phasic dopamine may be important for updating or resetting working memory traces, principally via D
2 receptors, while tonic dopamine may enhance stability of traces, principally via D
1 receptors
(10). Detailed biophysical models suggest that sustained D
1 activation (tonic dopamine) helps prevent “uncontrolled, spontaneous switches into high-activity states (i.e., spontaneous activation of task-irrelevant representations)”
(11,
12).
Since COMT catabolizes extracellular dopamine, while rapid intrasynaptic degradation relies on the dopamine transporter (phasic dopamine), the Met allele may enhance tonic dopamine but have reciprocal effects on phasic dopamine. We hypothesized that the Met allele would be of benefit for tasks requiring tonic activation (cognitive stability) but have adverse effects on tasks requiring phasic activation (cognitive flexibility).
Most tasks used in studies of COMT genotype have demands for both cognitive stability and flexibility. The Wisconsin Card Sorting Test requires cognitive stability to establish and maintain an appropriate response set and flexibility for the set-shifting aspect of the task. N-back working memory tasks require both maintenance (stability) and updating (flexibility) of memory representations.
In this study we used a computerized Competing Programs Task
(13) that requires alternation between two rules of responding—imitation and reversal. Acquisition and maintenance of the imitation rule requires cognitive stability, whereas cognitive flexibility is needed to switch rules in the reversal condition.
Method
Twenty-six subjects (18 men and eight women; 25 white, one Hispanic; mean age=41.4 years [range=30–52]) with schizophrenia (N=21) or schizoaffective disorder (N=5) completed the Competing Programs Task
(13). Each subject provided written informed consent after receiving a complete description of the study, according to the guidelines of the Institutional Review Board. At the time of testing, subjects were clinically diagnosed, stable inpatients being treated with a variety of antipsychotic medications sometimes in combination with adjunctive mood stabilizers or antidepressants. Severity of symptoms was not formally assessed at the time of testing.
The Competing Programs Task requires subjects to respond with one or two key presses after seeing one or two visual/auditory cues (one or two squares presented simultaneously with the same number of tones). During blocks 1 and 3 (imitation), the subject must produce the same number of key presses as stimuli; during blocks 2 and 4 (reversal) the subject must press once for two stimuli and twice for a single stimulus. Each block ends (and the rule changes) after either a maximum of 20 trials or a criterion (eight consecutive correct responses) is reached. The subject must deduce the response rules from trial-by-trial feedback. COMT genotyping was performed as described previously
(14).
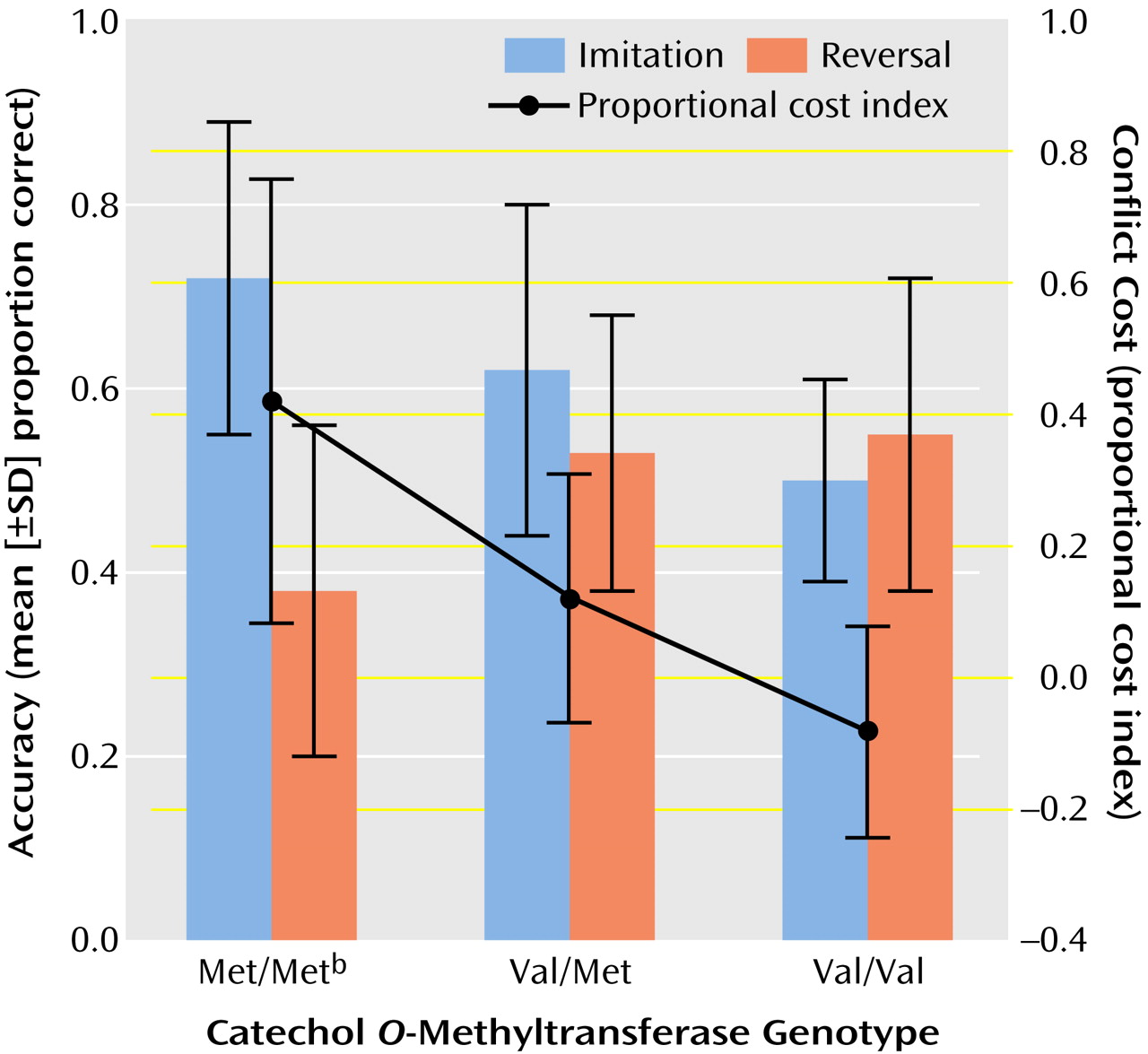
Accuracy measures included trials to criterion and proportion correct. We also calculated a proportional cost index
(15) to measure performance decrement related to shifting in the response rules from imitation to reversal: (proportion correct [imitation] – proportion correct [reversal]) / proportion correct [reversal].
Results
There were eight COMT Met homozygotes, six Val homozygotes, and 12 heterozygotes. The effect of genotype on reaction time was significant (F=4.8, df=2, 23, p<0.02; partial η2=0.29); reaction time was slower in Val than Met homozygotes (p<0.05, least significant difference test).
The effect of response rule (imitation/reversal) on proportion correct was significant (F=10.2, df=1, 23, p=0.004; η
2=0.31; imitation better than reversal), as was the response rule-by-genotype interaction (F=7.2, df=2, 23, p=0.004; η
2=0.39). Met homozygotes had greater accuracy than Val homozygotes for imitation responses but not reversal responses (
Figure 1). The response rule-by-genotype interaction was also significant for trials to criterion (F=4.4, df=2, 23, p<0.03; η
2=0.28). Trials to criterion was lower for Met homozygotes than Val homozygotes for imitation blocks only (t=–2.2, df=23, p<0.05). There were significant differences between genotypes in proportional cost index (F=7.92, df=2, 23, p=0.002; η
2=0.41). Met homozygotes showed the greatest sensitivity to conflict (
Figure 1).
Discussion
Consistent with the hypothesis, the Met allele of the COMT Val158Met polymorphism was associated with better cognitive stability but poorer cognitive flexibility. Met homozygotes showed better acquisition of the imitation rule but greater sensitivity to conflict when the response rules shifted from imitation to reversal. Since Met homozygotes had poorer absolute (not just relative) scores in the reversal condition, the increased “cost” of shifting does not appear to be an artifact of better performance in the imitation condition.
These findings suggest it may be plausible to define more precisely the phenotype most relevant to COMT genotype. In previous studies, COMT genotype accounted for 4%
(4,
16) to 11% (3) of the variance in performance. In this study, COMT genotype accounted for 28%–41% of the variance in performance on the Competing Programs Task.
Our results are consistent with the hypothesis that the Met allele leads to increased tonic dopamine activity, with general benefits for cognitive stability, but costs in the capacity to flexibly alter behavioral programs. This may help explain how the Met allele can be associated both with better cognitive performance and increased aggression. Further investigation of COMT effects may benefit from incorporating methods distinguishing cognitive stability from plasticity.
While these preliminary results may offer proof of concept, replication is clearly needed. Future research particularly needs to examine larger and more fully characterized samples and to look for possible genotype-by-treatment interactions. Given these caveats, we hope the findings may be useful in future attempts to refine the phenotypes associated with the COMT Val158Met polymorphism.