The neural underpinnings of memory can be studied with functional neuroimaging. A review concluded that the prefrontal cortex and hippocampus are most consistently implicated in schizophrenia
(5). Hemispheric asymmetry models have emphasized the importance of the left prefrontal cortex to episodic encoding and semantic retrieval and the right prefrontal cortex to episodic retrieval
(6). Subsequent studies supported a distinct role for the right anterior prefrontal cortex (Brodmann’s area 10) during episodic retrieval but found less evidence of hemispheric and task specificity for the dorsolateral prefrontal cortex (Brodmann’s area 9, 46)
(7,
8). The left dorsolateral prefrontal cortex appears to be involved in semantic organization during encoding
(9), and several prefrontal cortex subregions show functional overlap across working, episodic, and semantic memory
(10,
11). The importance of the hippocampus to memory was established in early studies of clinical pathology and was supported by functional imaging that attributed hippocampal function to encoding and retrieval, novelty detection, and successful retrieval
(12,
13).
Several investigators suggested that the reciprocal relationships between the prefrontal cortex and the mesial temporal lobe in normal cognition
(14) may be disrupted or reversed by schizophrenia
(15,
16). For example, in a verbal fluency study, investigators reported less prefrontal cortex activation and greater temporal lobe activation in patients than in healthy comparison subjects
(17). Other studies reported hippocampal overactivation in patients during baseline assessment and prefrontal cortex overactivation rather than hippocampal recruitment for recognition of words that had undergone deep semantic processing versus words that had undergone shallow perceptual processing
(18,
19). A subsequent study of the hippocampus involving word encoding and recognition also found less activation in patients than in healthy comparison subjects during both stages of processing
(20).
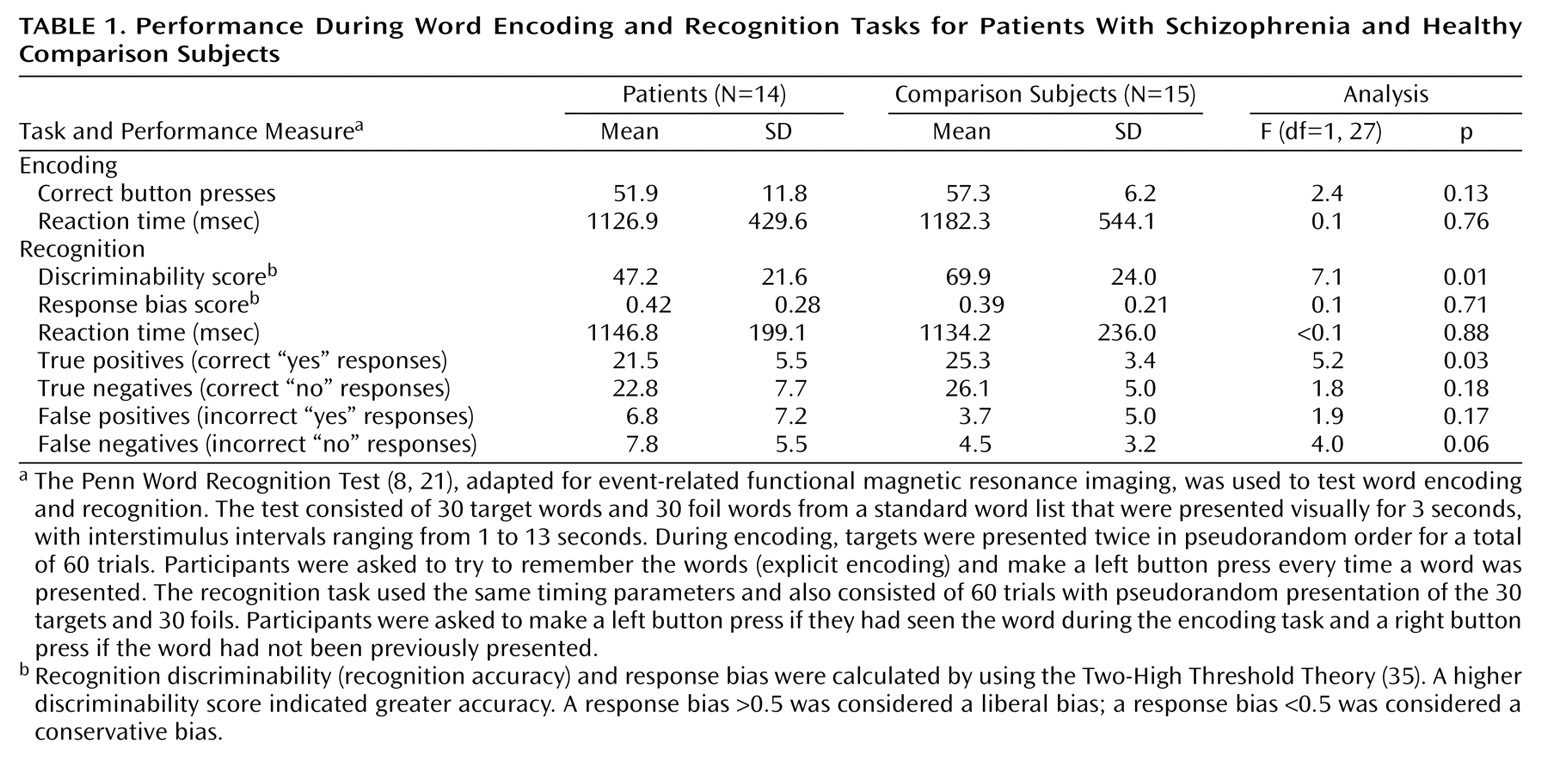
The purpose of the current study was to examine frontotemporal function in schizophrenia by using a verbal episodic encoding and recognition paradigm that was previously established with positron emission tomography (PET)
(8,
21). In previous studies, we found reduced left prefrontal cortex activation during encoding and recognition in patients with schizophrenia. Left superior temporal activity was also reduced during encoding, while activation in the left anterior cingulate, left mesial temporal, and right thalamic regions was reduced during recognition. However, right anterior prefrontal cortex activation during recognition was preserved. Performance correlated with prefrontal cortex activation in healthy participants and with posterior activation in patients. One goal of the present study was to establish the reproducibility of the PET findings with functional magnetic resonance imaging (fMRI). More important, event-related fMRI enables examination of retrieval success. We hypothesized that the left prefrontal cortex regions are disrupted by schizophrenia during both encoding and recognition but that schizophrenia patients would not differ from healthy comparison subjects in activation in the right frontal pole during recognition. Retrieval success was hypothesized to be related to prefrontal cortex activity only in the comparison subjects.
Discussion
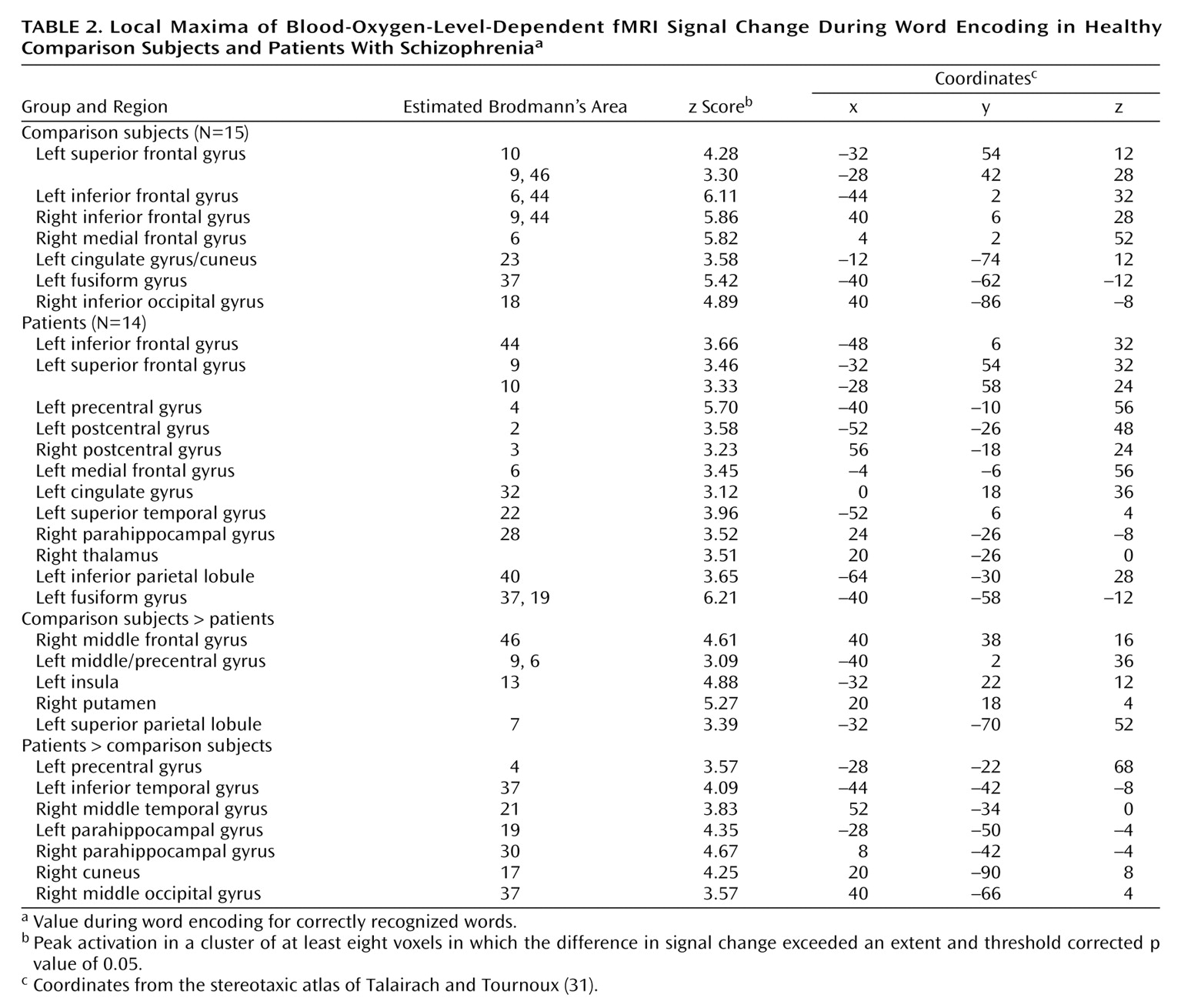
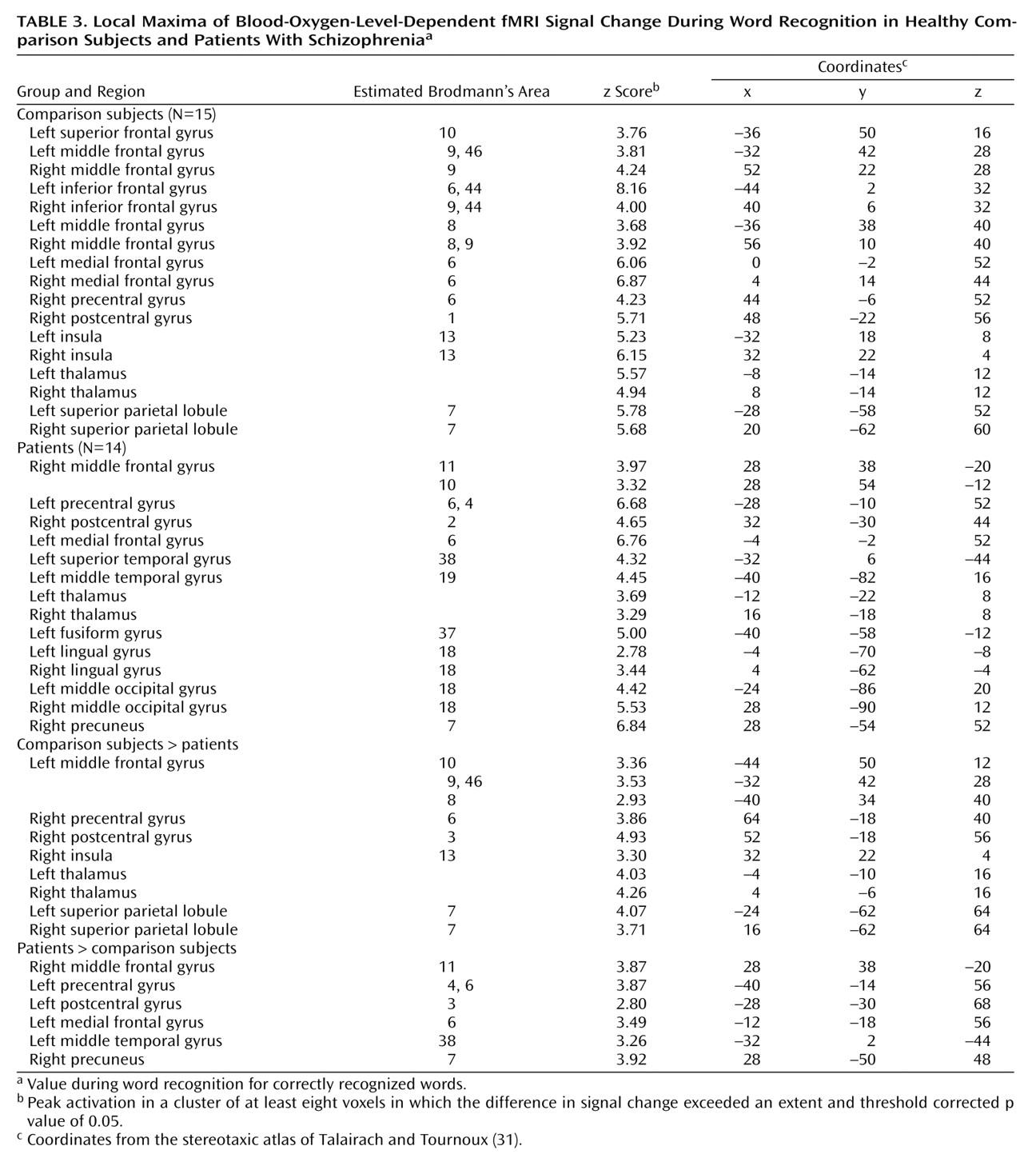
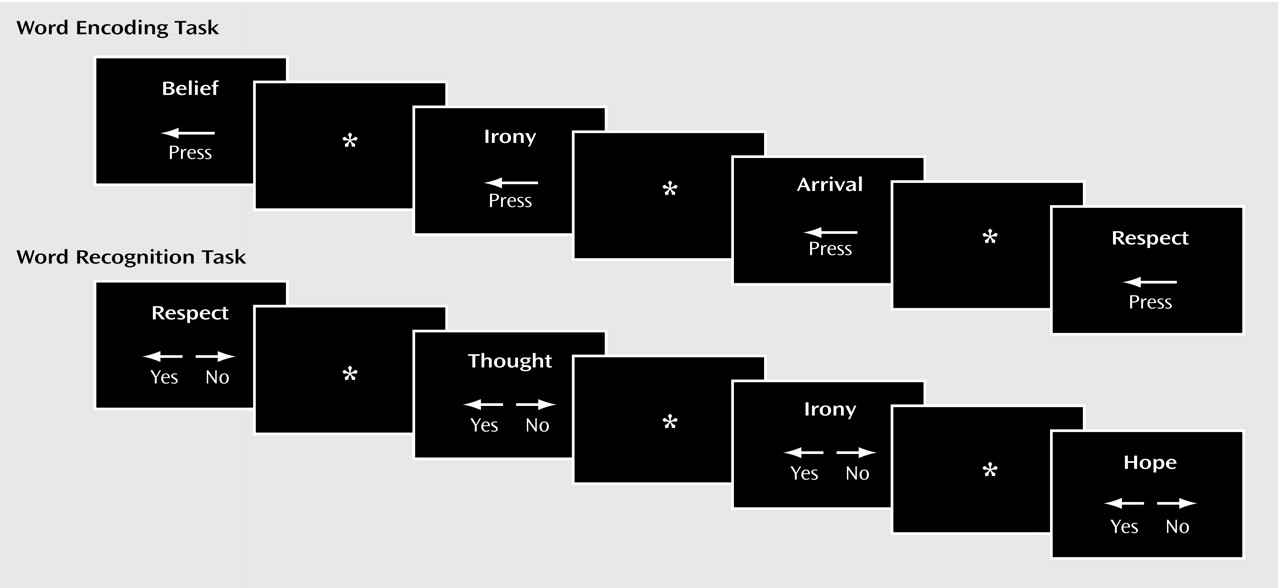
The current study reproduces our earlier PET findings
(21) of dorsolateral prefrontal cortex dysfunction in patients with schizophrenia during word encoding and recognition. Whereas dorsolateral prefrontal cortex dysfunction was restricted to the left hemisphere during PET, in the current study there was evidence in the patients of bilateral deficits during encoding, left hemisphere impairments during recognition, and right-sided dysfunction during retrieval success. In accord with earlier correlational results, the patients relied on a more distributed set of temporal-limbic and posterior regions, suggesting possible use of a language-based compensatory network
(44). As found previously, there was no group difference in right anterior prefrontal cortex activation during recognition, suggesting that the associated episodic retrieval mechanism
(45) is relatively intact in patients with schizophrenia. In contrast to our prior findings of less activation in the superior and mesial temporal cortex in patients with schizophrenia, the current study found that patients had greater temporal-limbic activation than comparison subjects during word encoding and recognition and during successful retrieval.
The dorsolateral prefrontal cortex was the frontal lobe area most clearly disabled by schizophrenia. Reproducing this result supports the importance of this region to episodic verbal memory and to schizophrenia pathophysiology
(21,
46). In contrast, there was no group difference in the left Broca’s area during encoding. Unimpaired activity in Broca’s area has been noted in other schizophrenia studies and attributed to intact verbal rehearsal in short-term memory
(47) (see reference
48 for exception). Discrepant effects of schizophrenia on the left dorsolateral prefrontal cortex and Broca’s activity may reflect encoding strategies found in patients. On the basis of self-report, the comparison subjects relied more frequently on associative semantic processing, which is linked with left dorsolateral prefrontal cortex function
(9). In contrast, the patients may have relied on verbal rehearsal mediated by Broca’s area
(49). Previous findings that patients with schizophrenia benefit from semantic encoding in a levels-of-processing paradigm
(18,
19,
50) suggest that failure to encode words semantically relates to difficulties in generating organizational strategies
(51) rather than to an inability to process words semantically.
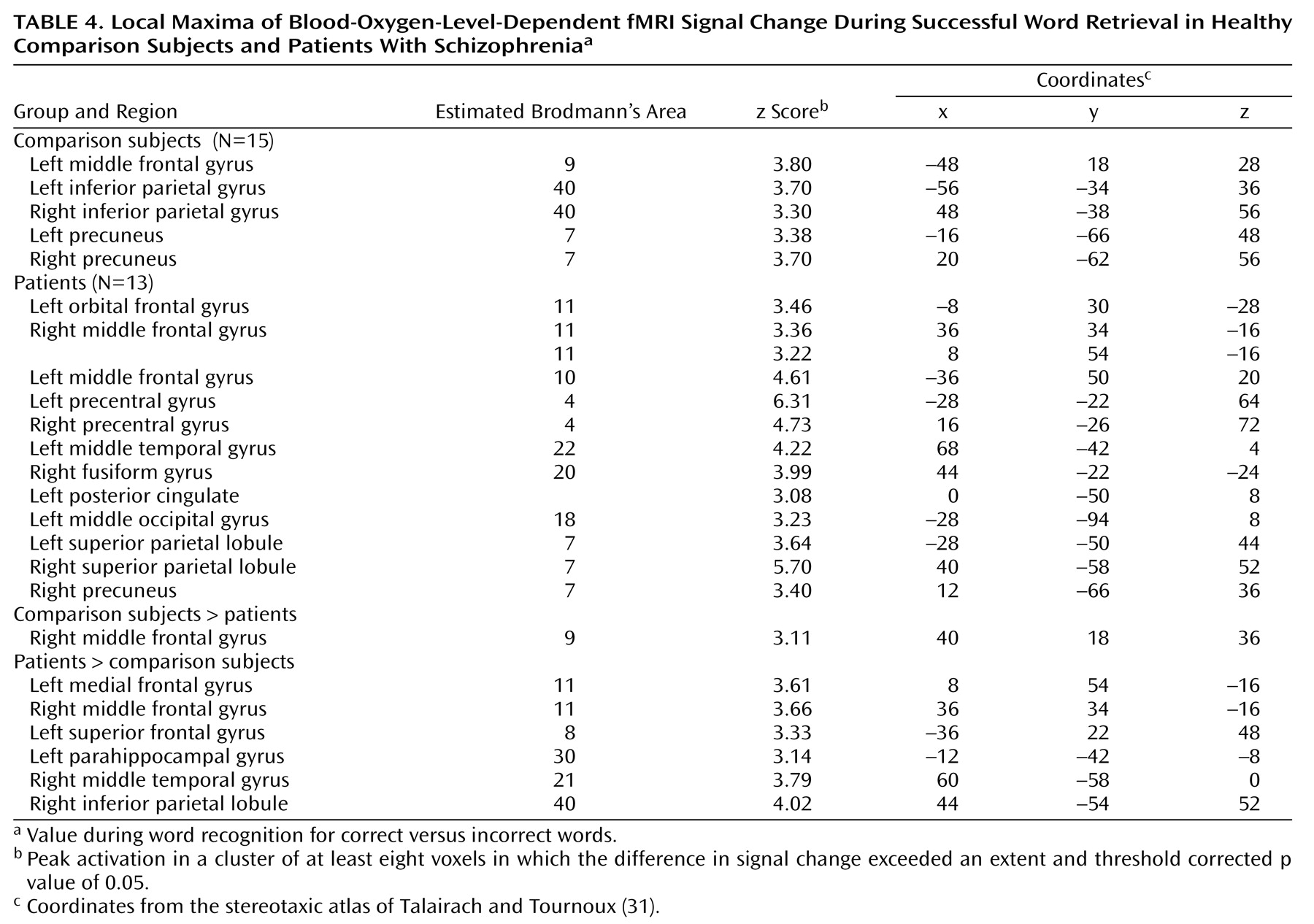
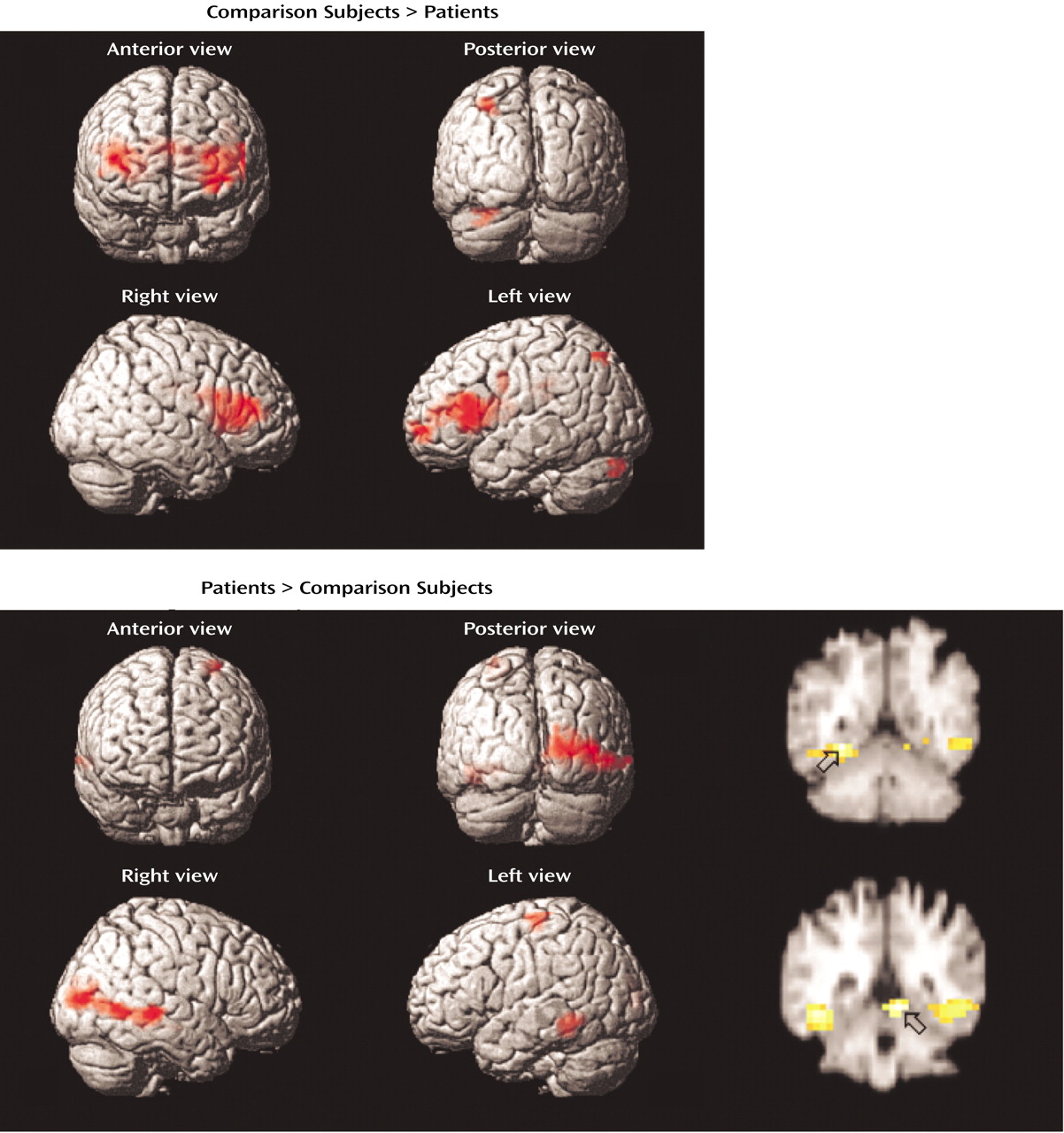
Dorsolateral prefrontal cortex dysfunction in patients extended to the right hemisphere during encoding and successful retrieval. This dysfunction was not found in our earlier PET study and should be viewed with caution. Recent event-related fMRI studies of successful retrieval in healthy individuals
(52,
53) have suggested that the right dorsolateral prefrontal cortex is important for postretrieval monitoring (e.g., monitoring the relevance of the retrieved information). Rather than engaging in this evaluative processing, the patients may have focused on processing the stimulus characteristics of the words, as they showed greater activation than the comparison subjects in sensorimotor and visual association areas.
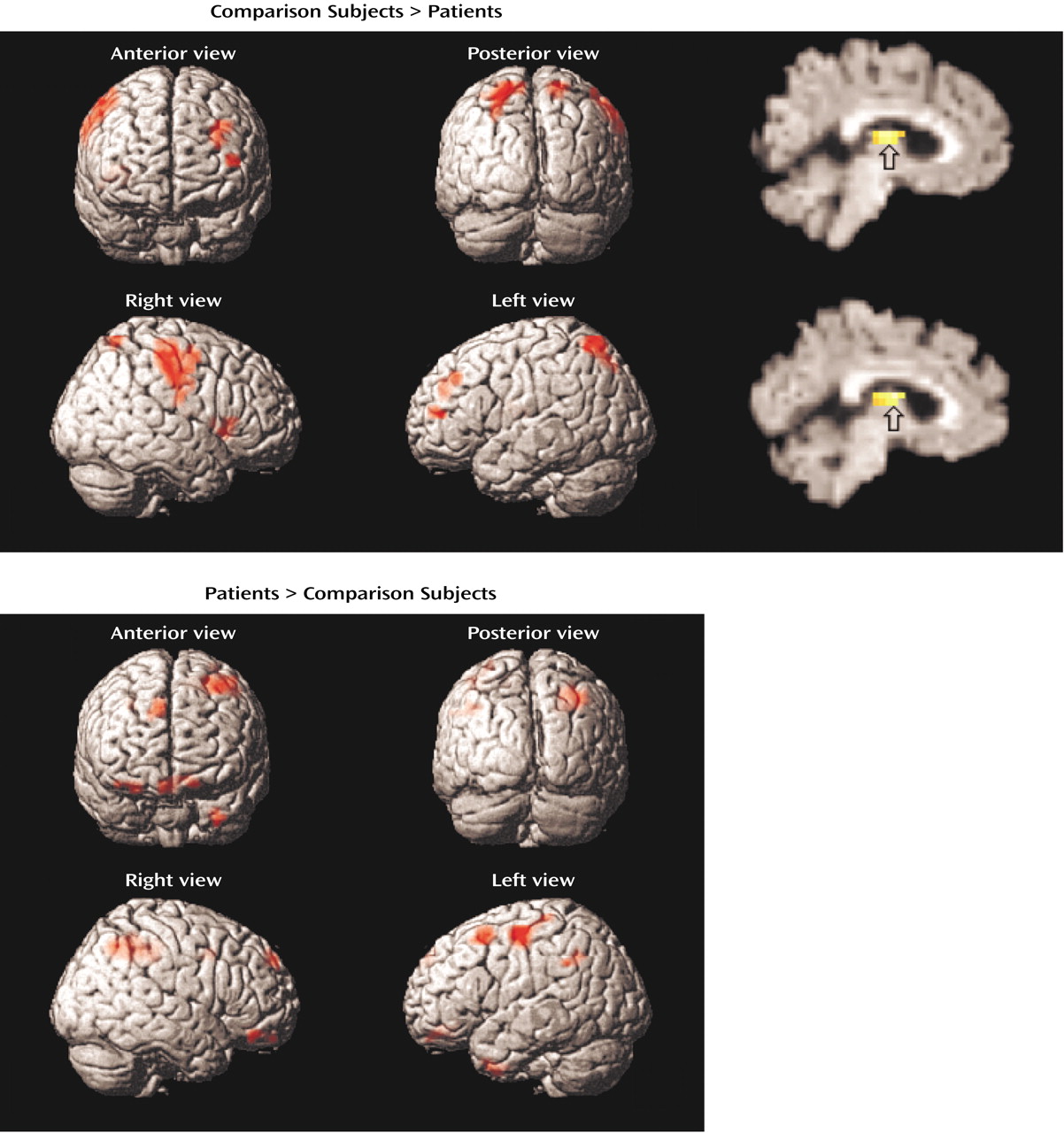
Within the prefrontal cortex, the patients also showed several areas of overactivation. Activity in left frontal pole was unimpaired in the patients during encoding. The patients also overactivated the orbitofrontal cortex during recognition and retrieval success. Heckers and colleagues
(18,
19) also found frontopolar and orbitofrontal overactivation in schizophrenia. Given patients’ better performance for words that had undergone shallow perceptual versus deep semantic processing, the investigators attributed this overactivation to greater retrieval effort
(18). This relationship also seems to exist in the patients in the current study, for whom the use of a less efficient encoding strategy may have led to greater retrieval effort. Regional group differences within the prefrontal cortex clarify the need to examine effects in specific subregions, as has been done in recent studies of working memory and episodic memory
(10,
11), and illustrate that not all aspects of prefrontal cortex are equally affected by schizophrenia. The dynamic nature of these abnormalities (both under- and overactivation) in patients suggests a disruption in the modulation of prefrontal cortex function in schizophrenia rather than a focal lesion in which the prefrontal cortex is unable to participate in memory processing.
Evidence of temporal-limbic overactivation in patients should be viewed with caution, as it does not replicate our earlier PET findings. However, there are several methodological reasons why hippocampal activity is not always detected by PET
(13), and the low temporal resolution of our PET method may account for this inconsistency. Nevertheless, dorsolateral prefrontal cortex underactivation and parahippocampal overactivation in patients suggest that the normal functional connectivity of prefrontal and temporal-limbic structures is disrupted or reversed by schizophrenia
(15,
16). The direction of this reversal has varied, with some studies showing the current pattern
(17,
54) and others showing less hippocampal activation and greater prefrontal cortex activation
(18,
19). Findings of both prefrontal cortex and hippocampal abnormalities should not be surprising, given the strong reciprocal connections between the hippocampus and the neocortex
(55). Neurodevelopmental hippocampal abnormalities in schizophrenia may mimic adult prefrontal cortex lesions since the hippocampus is a gateway for efferent pathways from the prefrontal cortex to the nucleus accumbens
(56).
This study had several limitations. Hippocampal activation was not seen in the comparison subjects, as in several previous studies
(18,
19). However, unlike prior studies, our study did not test recall and did not manipulate the encoding level. Eldridge and colleagues
(57) demonstrated that the hippocampus is engaged during retrieval only when subjects are required to make a conscious recollection. Lack of activation may reflect the use of a familiarity-based strategy. Another potential limitation is that patients were receiving medication. However, in our PET study we did not find any differences between medicated and unmedicated patients
(21), and in the current study medication dose did not correlate with performance. Others have also been unable to find medication effects on prefrontal or mesial temporal function in schizophrenia
(18,
19,
58) (see reference
59 for exception). Because of the small size of the medication subgroups in our study, we were unable to contrast fMRI results for patients who received typical versus atypical medications. Although performance did not differ between these medication subgroups, this issue deserves further attention, given findings suggesting differences in the effect of typical and atypical agents on cerebral blood flow in schizophrenia
(60). Finally, the current paradigm did not constrain encoding and retrieval conditions. We chose this approach because our initial goal was to assess reproducibility of a previously established paradigm. This lack of constraint had the advantage of revealing putative differences in strategic processes. Further studies employing levels-of-processing encoding conditions
(61) and source memory
(62) tasks will better clarify the role of encoding and retrieval strategies in episodic memory and related frontotemporal function in schizophrenia.