The dopamine D
2 receptor has long been of central interest in research on the pathophysiology of schizophrenia. Early positron emission tomography (PET) studies focused on the striatum, a region with a high density of D
2 receptors. Improvements in PET methodology now allow the examination of low-density dopamine D
2 receptor populations in several limbic and cortical regions in which structural or biochemical abnormalities have been reported in schizophrenia. In a recent analysis of cortical and subcortical regions, we found low radioligand binding to dopamine D
2 receptors in the anterior cingulate cortex of patients with schizophrenia and a correlation with positive symptom scores
(1).
On the other hand, functional abnormality of schizophrenia has also been discussed in terms of thalamic circuitry
(2). Imaging studies have consistently revealed smaller thalamic volumes in patients with schizophrenia as well as altered thalamic perfusion and metabolism
(2–
13). Neuropathological studies have found a reduction in the number of neurons in the mediodorsal nucleus of the thalamus in schizophrenia brains
(14–
17). Schizophrenia-associated neuronal loss has also been found in the pulvinar
(17). A synapse-related protein study reported that the thalamic abnormalities include synaptic disturbances
(18).
The thalamus was not included in early maps showing dopaminergic innervation in the rodent brain
(19). However, dopamine D
2 receptors have more recently been identified in the human thalamus in vitro
(20,
21) and in vivo using PET
(22–
24). The possibility that dopamine D
2 receptors in the thalamus are involved in the therapeutic actions of antipsychotics has been supported by PET studies that demonstrated dopamine D
2 receptor occupancy in the thalamus by antipsychotic drugs
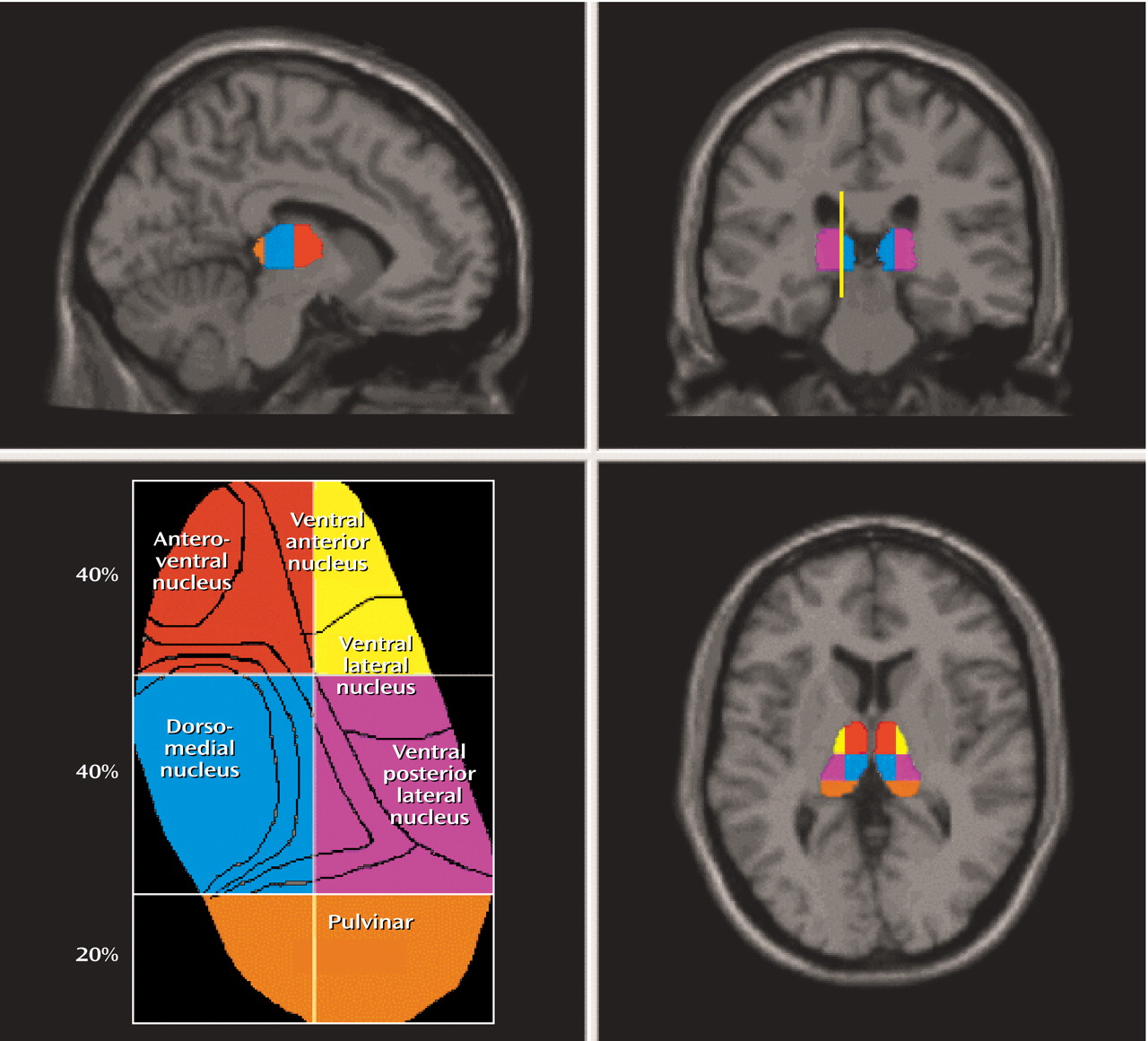
(25). Each of the major 23 subnuclei of the thalamus
(26) has a unique set of efferent and afferent projections with different functional implications. In a previous study, we found a tendency toward low density of dopamine D
2 receptors in the thalamus
(1). However, in that study we focused on the thalamus as a whole and did not take its detailed complexity into account.
The aim of the present PET study was to examine separately dopamine D
2 receptor binding in five major subregions of the thalamus. Ten neuroleptic-naive patients with schizophrenia and 19 healthy comparison subjects were examined with the radioligand [
11C]FLB457, a substituted benzamide with a very high affinity for dopamine D
2 receptors
(27). We then investigated whether there were correlations between subregional binding and psychopathology score as assessed with the Brief Psychiatric Rating Scale (BPRS)
(28).
Results
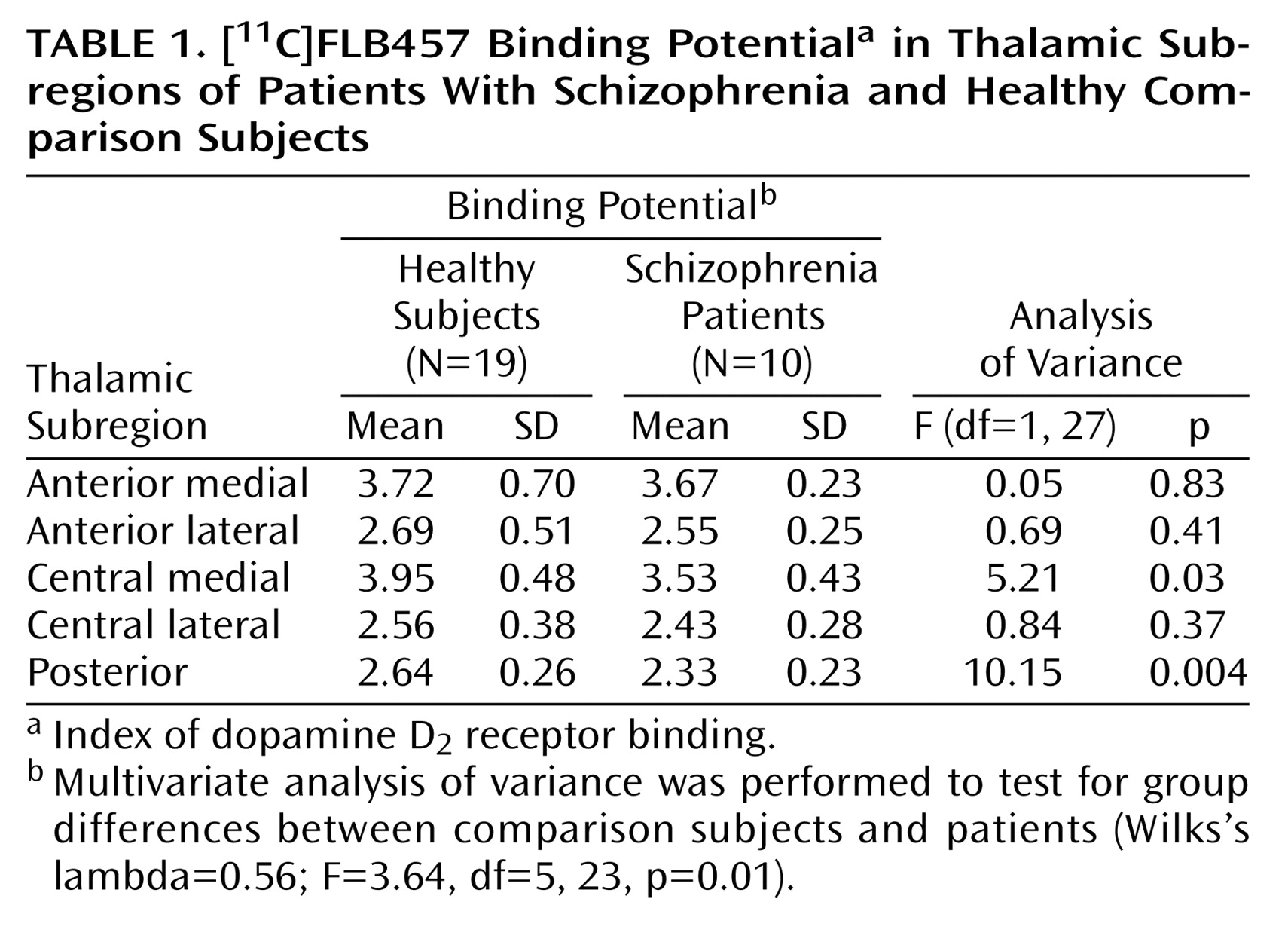
With regard to the binding potential values for [
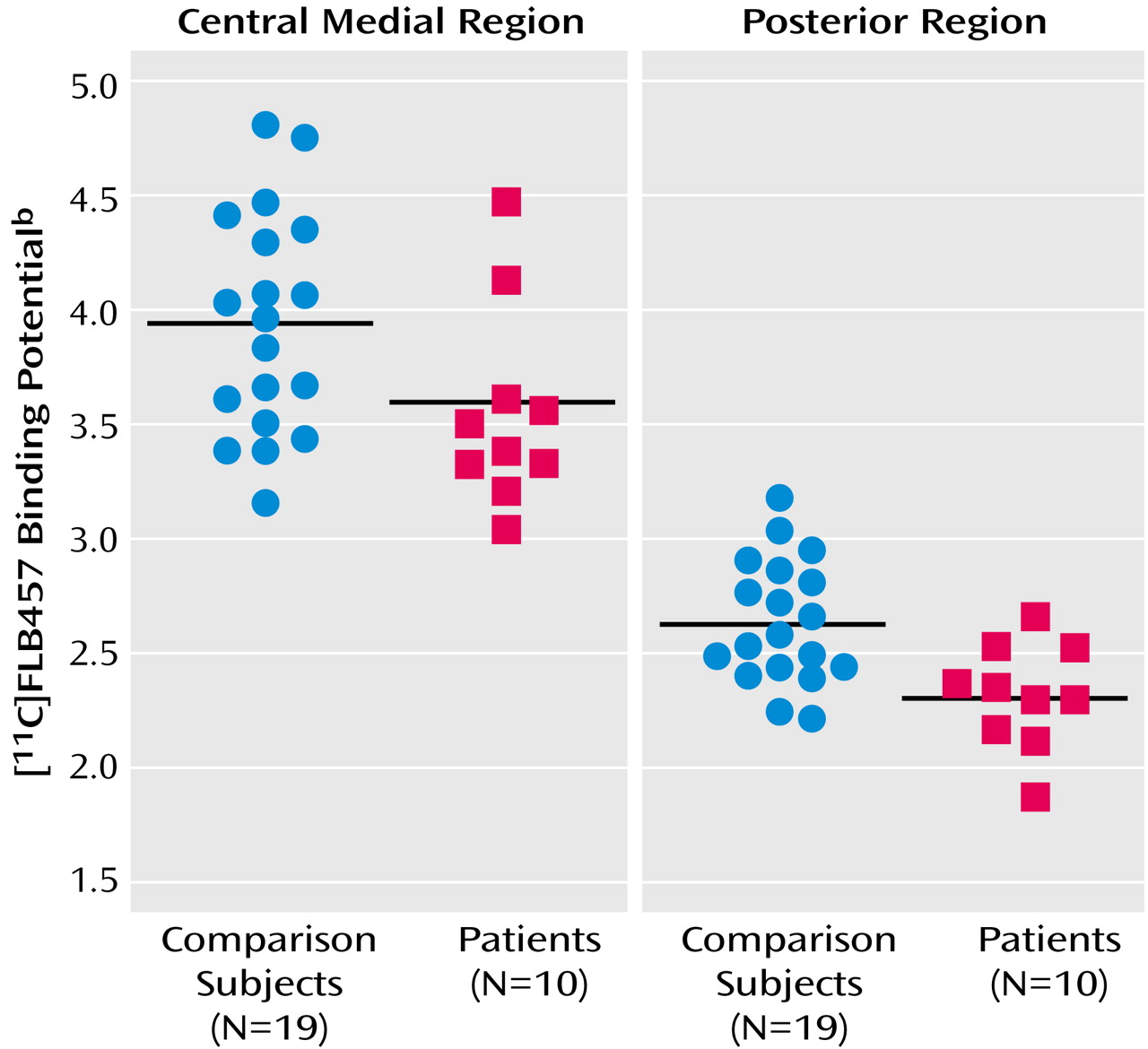
11C]FLB457 binding in the whole thalamus, there was no significant difference between the two groups (schizophrenia patients: mean=3.31, SD=0.33; comparison subjects: mean=3.54, SD=0.45) (t=1.45, df=27, p>0.15). However, multivariate analyses of the binding potential values in the thalamic subregions showed significant differences between patients with schizophrenia and comparison subjects. Follow-up ANOVAs revealed that the binding potential values in the central medial region and the posterior region were significantly lower in the patients (
Table 1,
Figure 2).
The influx ratio (R
1) for the whole thalamus (schizophrenia patients: mean=0.84, SD=0.50; comparison subjects: mean=0.87, SD=0.57) did not differ significantly between patients and comparison subjects (t=1.62, df=27, p>0.12) nor did it differ in any subregion (
Table 1). The volume of the whole thalamus was not significantly different between patients (mean=8.65, SD=1.16) and healthy subjects (mean=8.65, SD=1.19) (F=0.005, df=1, 26, p>0.94), and no significant difference was found for any of the thalamic subregions (
Table 1).
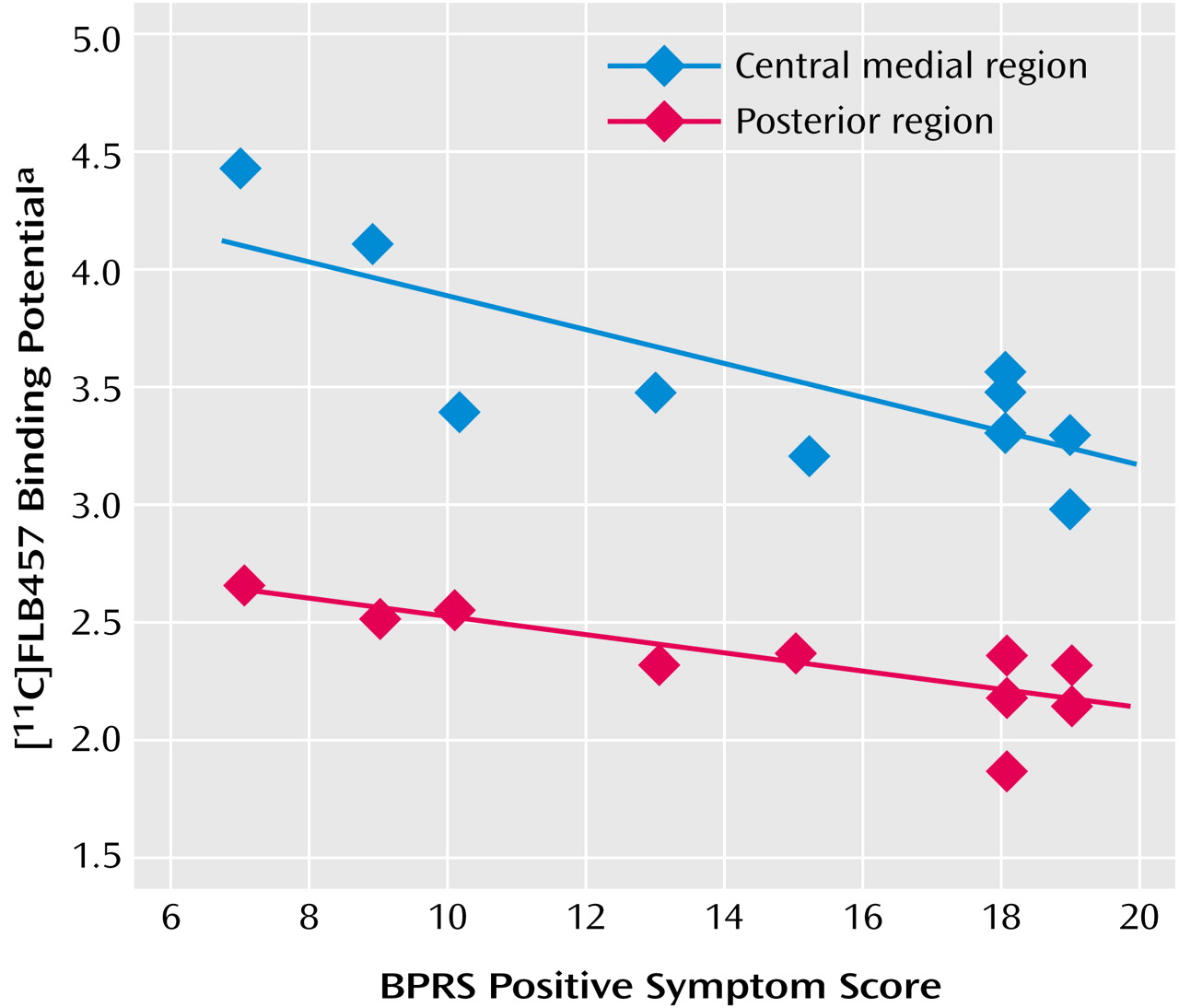
For the central medial region and the posterior region there was a statistically significant negative correlation between binding potential and positive symptom subscores on the BPRS (
Table 1,
Figure 3). There was no significant correlation between binding potential and the BPRS total scores or negative symptom subscore for any region. Further, no significant relationship was observed between regional binding potential values and the duration of illness, age, or parental socioeconomic status in comparison subjects and patients.
Discussion
In a previous PET study, we reported low dopamine D
2 receptor binding in the anterior cingulate in patients with schizophrenia and a statistical trend for low binding in the thalamus
(1). In the present study with a partly overlapping patient group, a more detailed analysis of the thalamus indicated low [
11C]FLB457 binding potential in the central medial and posterior subregions of the thalamus in patients with schizophrenia. Highly elevated levels of dopamine have been found postmortem in the thalamus of schizophrenia
(36), and the reduction in binding could be attributed to an increase in endogenous dopamine. However, our previous study indicated that extrastriatal [
11C]FLB457 was not sensitive to endogenous dopamine, as [
11C]FLB457 binding in the cortex and thalamus was not significantly affected by a 1-mg/methamphetamine challenge
(37). Therefore, the present findings may be attributed to the receptor density. A longitudinal study will be required to settle the issue of whether low density is acquired during the course of disease or whether it represents abnormal brain development
(38).
The subregions with low D
2 receptor binding comprise primarily the dorsomedial nucleus and pulvinar, two important components in circuitries previously suggested in the pathophysiology of schizophrenia
(5,
7). The thalamus has long been suggested to have a gating function that filters the sensory input to the cortex, thereby providing protection against sensory overload and hyperarousal
(39). Startle prepulse inhibition, a sensitive measure of sensory gating, has indeed been suggested as being related to dopamine neurotransmission in the thalamus
(40). Both animal and human studies have provided evidence that the mediodorsal thalamus has a particular role in the regulation of startle prepulse inhibition
(41–
43). In experimental studies, it has been shown that prepulse inhibition of startle can be disrupted after microinjection of the dopamine agonist apomorphine into the medial thalamus
(43). The dorsomedial nucleus has connections to the prefrontal cortex and anterior cingulate
(44). Both these regions have also been included in discussions on the gating function
(39). An abnormality of the dorsomedial nucleus in schizophrenia may be attributed to functional disturbances in any of the brain regions discussed in relation to sensory gating.
The other subnuclei with low binding potential are found in the pulvinar. This is one of several structures implicated in attentional processing and salience
(45–
47). The pulvinar has connections with the visual and auditory cortex as well as the prefrontal cortex and temporal association areas
(48). A primary abnormality in the pulvinar may thus induce unusual associations and sensory disturbances in schizophrenia
(26). Taken together, these two regions may have a functional role consistent with several of the disturbed functions in schizophrenia and underlie the correlation with BPRS positive symptom score.
The observation of a significant negative correlation between subregional binding potential and positive symptom score is similar to that previously reported for [
11C]FLB457 binding in the anterior cingulate. The anterior cingulate has direct connections with the dorsomedial nucleus and the pulvinar
(48–
50). When we examined the relationship between dopamine D
2 receptor binding in the central medial and posterior regions of the thalamus and that of the anterior cingulate using the Pearson correlation method, we found a significant interrelationship between those regions in patients with schizophrenia (p<0.05). The abnormality shown in the thalamic subregions might have a similar background to that in the anterior cingulate. However, the cellular localization of D
2 receptors in the thalamic subregions that might allow speculation about the altered regulatory function of interneurons with D
2 receptors has not yet been determined, as discussed in the previous study
(1).
Reduced regional blood flow has been reported in the thalamus of schizophrenia
(51). However, the reduction of binding potential is unlikely to be a result of altered blood flow, since the R
1 value did not differ significantly between the healthy subjects and patients. While morphological changes have been reported in the thalamic mediodorsal nucleus and pulvinar in schizophrenia
(7,
8), and this can affect binding potential, no significant difference was observed in the volumes of thalamic subdivisions, including the central medial and posterior regions, between our examined patient and comparison groups. The relatively moderate severity and short duration of illness in our patient group may explain the lack of volume change
(52,
53). In any case, low dopamine D
2 receptor binding can therefore not be attributed to reductions in gross brain anatomy.
There are several confounding factors in this study. First, the number of patients was small, raising the question of adequate statistical power, and thus it cannot be ruled out that a larger study population might reveal that the binding potential values of other thalamic subregions and volumetric measurements will also show significant differences. In addition, [
11C]FLB457 has high affinity not only for dopamine D
2 receptors but also for dopamine D
3 receptors
(27). Dopamine D
3 receptors are distributed mainly to the ventral striatum and the islands of Calleja in the postmortem human brain, but they have as yet not been distinctly identified in the thalamus
(54–
56). Thus, there is a possibility that our findings could be partly explained by the reduction of dopamine D
3 receptors, but this will have to await the outcome of future studies on the amount of dopamine D
3 receptors in the thalamus. Another factor is that psychopathology was assessed by the 18-item BPRS, but this scale mainly measures the affective component of the negative symptoms and does not cover well the additional components that identify cognitive, anergic, and social dimensions
(57).
Finally, our measurement of thalamic subdivisions has several limitations. We were unable to delineate and employ an intrathalamic marker as a consistent landmark for our regional subdivisions. Rather, we relied upon approximate percentage-based divisions of the total thalamic area as a means of dividing the thalamus. This automated method reduced some of the subjectivity and systematic bias involved in defining subthalamic areas with limited resolution imaging. However, without manual editing, the assumptions that all thalamic nuclei are consistently represented by these rigid subdivisions cannot be assured, and the volume of each subdivision would not be comparable with data from carefully edited volumetric studies.