Abnormal feedback loops within cortical-striatal-thalamic-cortical circuits have been hypothesized to play a key role in the pathophysiology of OCD. Specifically, a defect in the basal ganglia’s ability to provide sensory gating by filtering and suppressing inputs from cortical regions has been considered important to OCD phenomenology
(4–
8). Basal ganglia abnormalities have been linked to OCD from studies that have demonstrated obsessive-compulsive symptoms in individuals with known dysfunction in these regions
(4). Several prior structural magnetic resonance imaging (MRI) studies of the caudate nucleus have reported no volume differences between patients and healthy volunteers
(9–
13), although others have reported larger
(14) or smaller
(15) volumes in patients. Studies of other parts of the corpus striatum, including the putamen
(13,
16) and globus pallidus
(16–
18), in OCD have also yielded mixed findings.
Neurobiological models of OCD have also emphasized abnormalities in the frontal lobes
(6–
8). In particular, the anterior cingulate basal ganglia-thalamocortical circuit
(19–
21) has been hypothesized to play a key role in compulsive behavior
(4). Evidence from neuropsychological
(22), structural MRI
(23), and functional neuroimaging
(24,
25) studies has implicated anterior cingulate abnormalities in the pathogenesis of OCD. Moreover, a developmentally mediated network dysplasia involving the basal ganglia and anterior cingulate has been hypothesized to disrupt brain functions that mediate ongoing purposive behavior in OCD
(13). In contrast to the attention focused on the anterior cingulate gray matter, there has been little systematic study of the anterior cingulate white matter in OCD, despite its strong connections with other brain regions that have also been implicated in the pathophysiology of OCD, including the ventromedial caudate, putamen, and nucleus accumbens.
Results
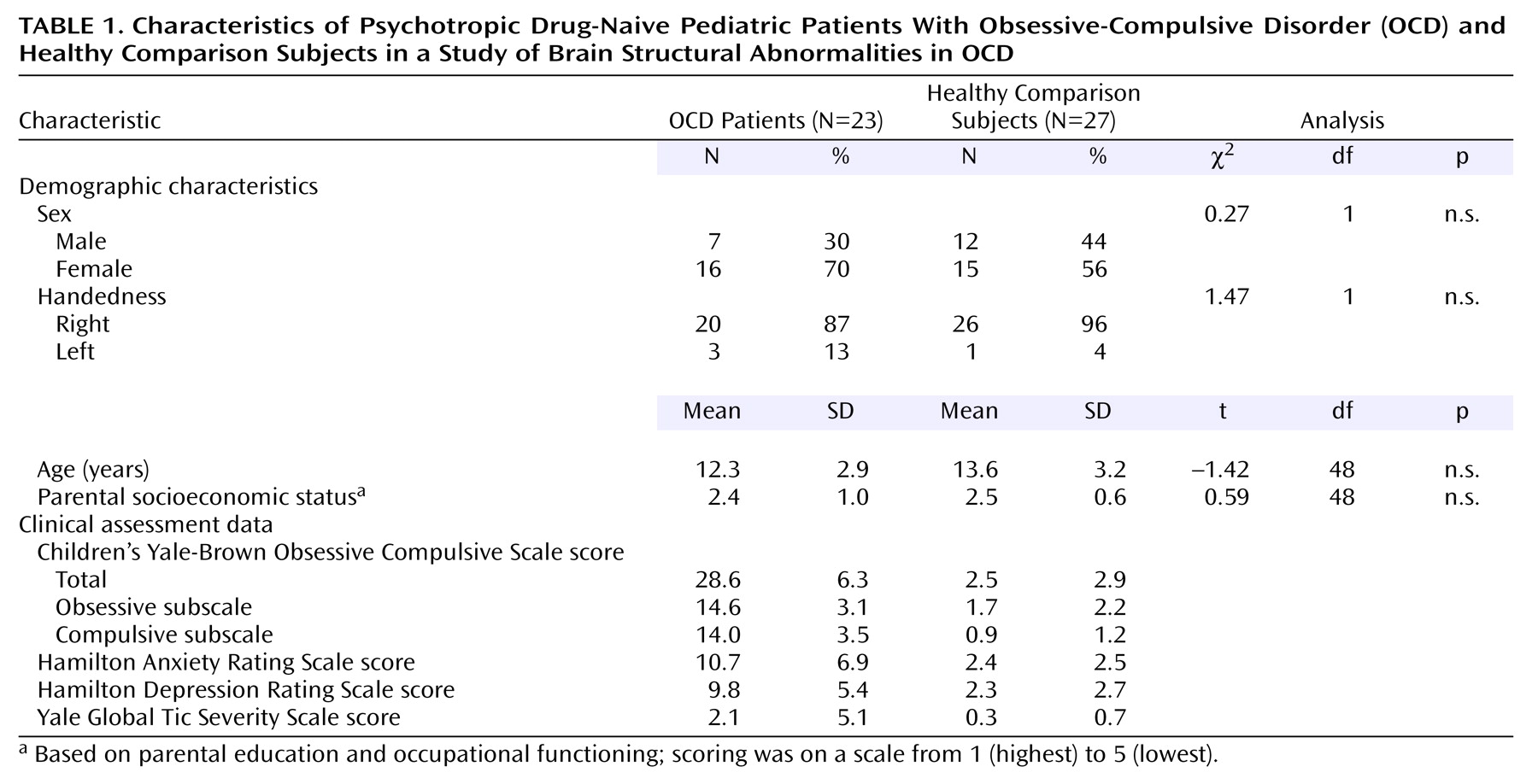
The characteristics and clinical assessment data for the psychotropic drug-naive patients with OCD and the healthy comparison subjects are summarized in
Table 1. All patients had significant dysfunction as measured by the Children’s Yale-Brown Obsessive Compulsive Scale score (mean=28.6, range=17–40). The patients and the comparison subjects did not differ significantly in distributions of age, sex, parental socioeconomic status, or handedness (all p>0.05). The groups did not differ significantly in intracranial volume (patients: mean=1188 cm
3, SD=109; healthy volunteers: mean=1169 cm
3, SD=112) (t=0.60, df=48, p=0.55).
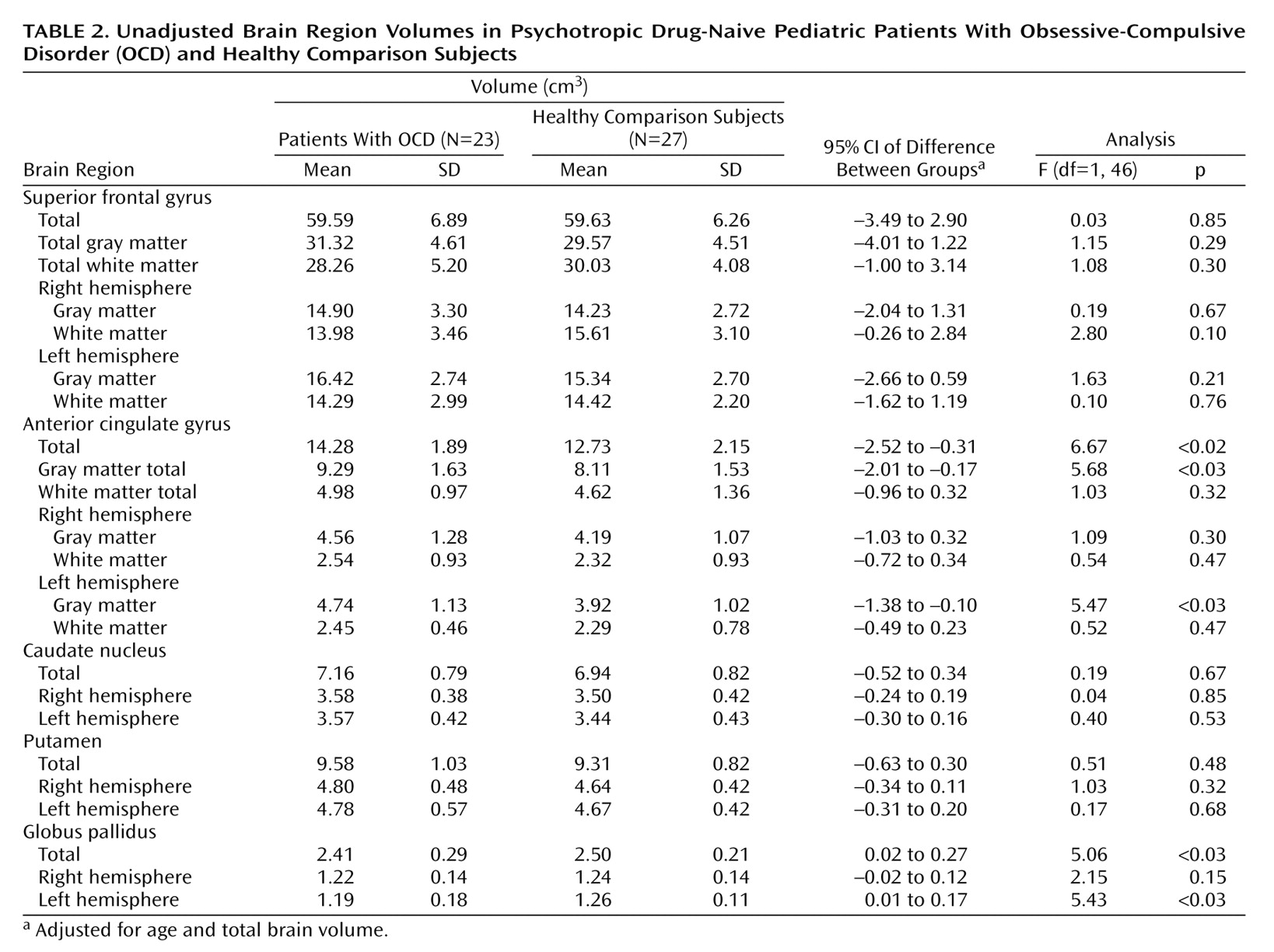
Because the group-by-hemisphere interaction was not significant for any of the brain structure volumes, right and left hemisphere volumes were pooled for the analyses. In addition, given that the diagnosis-by-sex interaction was not significant for any of the brain structure volumes (p>0.05 for all analyses), sex was not included in the final model. The MANCOVA for the brain structure volumes revealed that the overall main effect of diagnosis was significant (Wilks’s lambda=0.69, F=2.56, df=7, 40, p<0.03), indicating that the volumes differed between the groups. Univariate tests revealed that the patients had more anterior cingulate gyrus gray matter (F=5.68, df=1, 46, p<0.03) and smaller volumes of the globus pallidus (F=5.06, df=1, 46, p<0.03), compared to the healthy volunteers. Significant group differences were still evident when the effects of depression and anxiety were controlled. In addition, patients who had a comorbid diagnosis (N=12) did not differ significantly from patients who had OCD as their sole diagnosis (N=11) in either anterior cingulate gyrus gray matter or globus pallidus volumes (p>0.05). The mean brain structure volumes are presented in
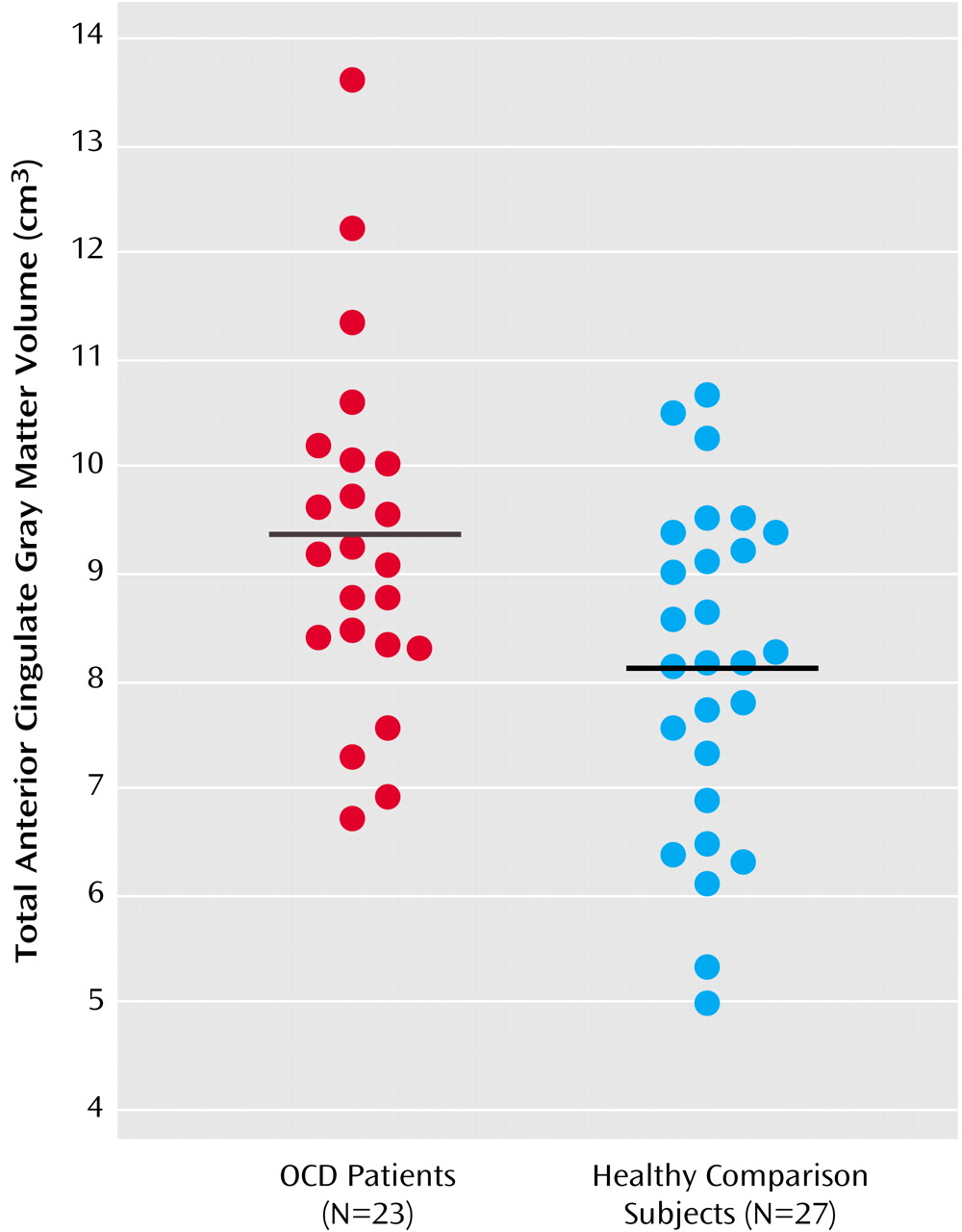
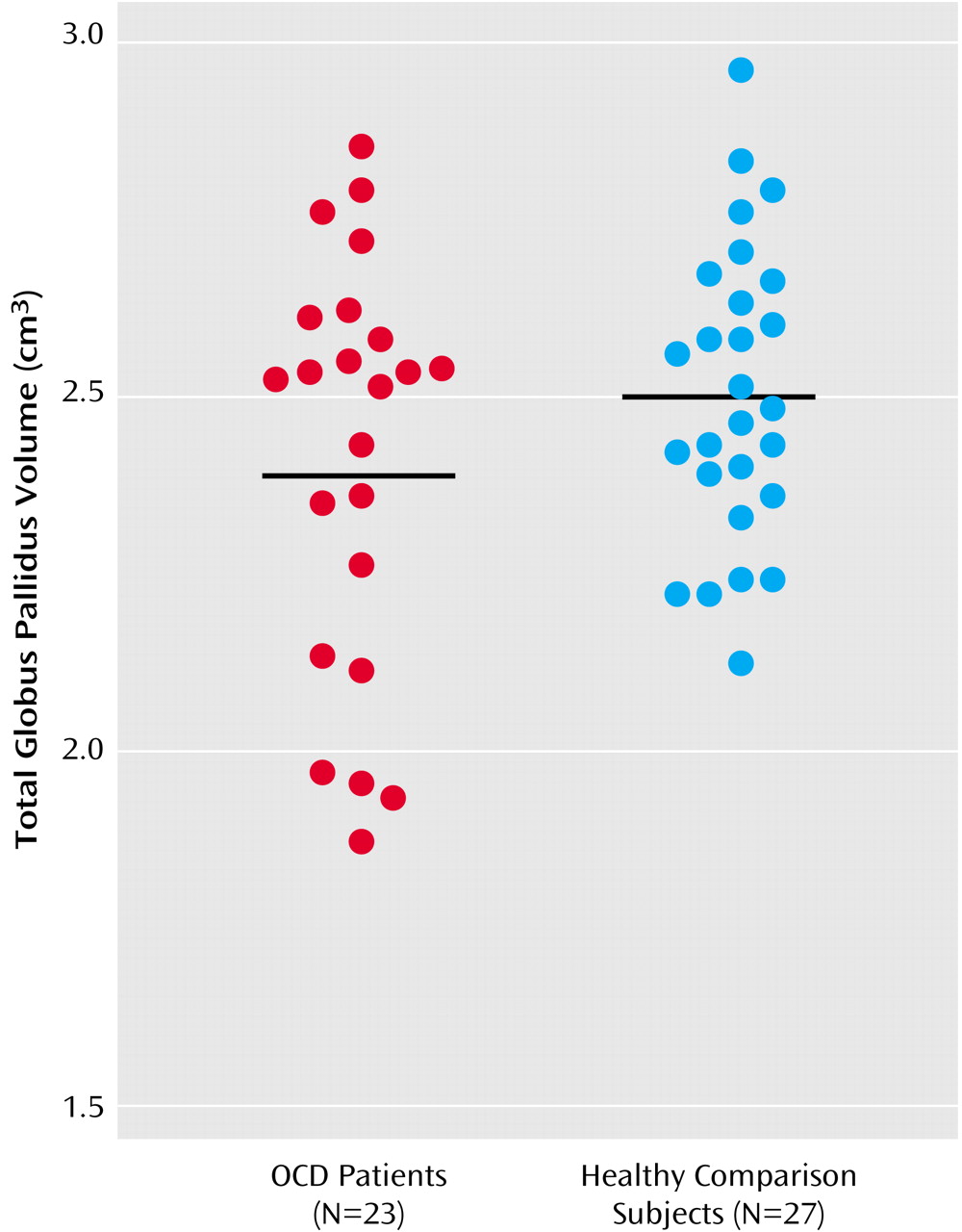
Table 2, as are the adjusted 95% confidence intervals for the differences between group means (the mean for the healthy comparison group minus the mean for the patient group). The distributions of the unadjusted anterior cingulate gyrus gray matter and globus pallidus volumes by group are illustrated in
Figure 1 and
Figure 2, respectively.
We found no significant correlations of Children’s Yale-Brown Obsessive Compulsive Scale, Hamilton depression scale, or Hamilton anxiety scale scores with either anterior cingulate gray matter volume or globus pallidus volume in the patients (p>0.05). However, globus pallidus and total anterior cingulate gyrus volumes were positively correlated in the healthy comparison subjects (r=0.51, df=27, p=0.007) but not in the patients (r=–0.07, df=23, p=0.75).
Discussion
Our findings provide additional evidence for brain structural abnormalities in the pathophysiology of OCD without the potentially confounding effects of prior psychotropic drug exposure and chronicity of illness. To our knowledge, these findings provide the first controlled MRI evidence of structural alterations in the globus pallidus in OCD. In addition, using methods for cortical parcellation based on the sulcal anatomy, we found that volumetric abnormalities in the anterior cingulate gyrus are specific to the gray matter, at least at the gross anatomic level, in patients, compared to healthy volunteers.
Few MRI studies have investigated the globus pallidus in OCD. Although Jenike et al.
(16) did not identify any globus pallidus abnormalities in a group of 10 female adults with OCD, the small number of subjects may have limited the statistical power. Sampling differences, including differences in prior pharmacologic exposure, could also account for discrepant findings. Our findings are consistent with case reports linking globus pallidus pathology to obsessive-compulsive phenomenology
(17,
18). Similarly, Laplane et al.
(40) reported obsessive-compulsive behavior in seven patients with basal ganglia lesions, five of whom specifically had globus pallidus pathology. Moreover, abnormal metabolic rate in the globus pallidus has also been implicated in the pathophysiology of OCD
(41). Prior reports of higher than normal globus pallidus volume in OCD have been linked to streptococcal infection
(42) and higher antibody titers
(43); these findings are consistent with the hypothesis of an inflammatory autoimmune response. Thus, these earlier findings may not be directly comparable to our findings. Further investigation of the relationship between brain structure volumes and indices of streptococcal infection is warranted.
The globus pallidus has been considered to play an important role in neurobiological models of OCD
(5,
6). In particular, this region has considerable afferent connections from other regions implicated in the pathophysiology of OCD, including the thalamus and striatum
(44). Moreover, the orbital frontal lobe has extensive connections with the ventromedial caudate and nucleus accumbens, which also project to the ventromedial globus pallidus. In the model by Baxter et al.
(6), the orbital frontal lobe is hypothesized to produce “worry” outputs that drive OCD-relevant circuits in the caudate nucleus, thereby increasing inhibitory output to other brain regions, including the globus pallidus. Similarly, Modell et al.
(5) have proposed that dysfunction within a fronto-striato-pallido-thalamo-frontal circuit could contribute to the inhibitory mechanisms (i.e., control or loss of control) observed in OCD.
Few structural MRI studies have investigated anatomically relevant frontal lobe subregions in OCD, which may be an important consideration, given that the frontal lobes are both structurally and functionally heterogeneous. Several prior studies in OCD did not report group differences in volumetric measures of the entire prefrontal cortex
(13,
15). Jenike et al.
(16) reported larger operculum volumes in 10 female patients, compared to 10 healthy volunteers. For the same group of subjects, Grachev et al.
(45) reported that the volumes of 10 frontal cortical parcellation units (including the right inferior frontal pars triangularis and the right midfrontal cortex) were larger in patients, compared to healthy volunteers. One advantage of the methods described herein is that they are based on the sulcal anatomy and, thus, may provide greater cytoarchitectonic validity, compared to methods that use invariant landmarks that are not on the cortical surface, such as the corpus callosum. It should be acknowledged, however, that the size of cytoarchitectonic fields can vary among individuals and that these variations may not map neatly onto the sulcal anatomy of the cortex
(46). Nevertheless, it is noteworthy that no group differences were observed in a frontal “comparison” region—the superior frontal gyrus—thus demonstrating some degree of specificity of gray matter anatomic pathology to the anterior cingulate gyrus in OCD.
The finding of more anterior cingulate gyrus gray matter in patients is consistent with reports of functional abnormalities in this region
(24,
25,
47,
48). Recent functional magnetic resonance imaging data suggest that hyperactivity in the anterior cingulate may be associated with abnormal conflict detection as part of an overactive action monitoring system in OCD
(49). Moreover, animal studies suggest that the anterior cingulate plays a key role in reward expectancy
(50). Thus, this region may have particular relevance to behavioral theories regarding OCD pathogenesis. More anterior cingulate gray matter in OCD could reflect a neurodevelopmental abnormality that possibly involves neuronal pruning
(23), although it is also conceivable that hyperfunctioning in the anterior cingulate could lead to increased gray matter volume.
Our finding of a larger amount of anterior cingulate gray matter in patients replicates prior findings
(23) in a new group of psychotropic drug-naive patients and by means of different methods. Our findings extend this prior work by demonstrating that structural alterations in the anterior cingulate gyrus are specific to the gray matter, at least at the gross anatomic level, given the nonsignificant group differences in anterior cingulate white matter volume. If anterior cingulate abnormalities are specific to the gray matter in OCD, this finding may partly explain why we did not observe group differences in volumes of the total (gray plus white) anterior cingulate gyrus in a previous study
(37), although the majority of patients in that study had been exposed to psychotropic medications, thus making comparisons with the present findings difficult. In a study by Kim et al.
(26), however, that included adults with OCD who had all remained psychotropic drug-free for at least 4 weeks before the MRI scan, no gray matter abnormalities in the anterior cingulate gyrus were identified in patients by means of statistical parametric mapping, suggesting that methodologic and/or sampling issues (i.e., pediatric versus adult patients) may be important considerations in interpreting the study findings. However, the patients in the study by Kim et al.
(26) may have required more time for medication washout before specific structural abnormalities in the anterior cingulate could be detected.
Taken together, the findings of abnormalities in both the globus pallidus and the anterior cingulate gyrus in the patients are consistent with the model of OCD pathophysiology described by Rapoport and Wise
(4). In this model, striatal cell groups act as signal detectors either for sensory-derived inputs from the cortex or for “internal motivation” inputs from the cingulate cortex. Sensory input to the striatum causes an innately programmed cell group to recognize the input and stop the tonic discharge of pallidal cells. Removal of thalamic inhibition releases thalamocortical circuits, thus leading to a normal behavioral response. If signals from the cingulate cortex to the striatum converge on the same pallidal cell group responsible for the species-typical behavior, then cingulate hyperactivity could conceivably activate this circuit without concomitant sensory input or motivation to perform the behavior, thus causing it to be completed compulsively. The finding of a positive correlation between globus pallidus and anterior cingulate gyrus volumes in the healthy volunteers, but not in the patients, in the present study is consistent with the hypothesis that a defect in connectivity of frontal-subcortical circuitry may play a role in the pathophysiology of OCD.
Similar to some MRI studies
(9–
13), but in contrast to others
(14,
15), our study did not find structural abnormalities in the caudate nucleus of patients with OCD. Differences among studies—including differences in delineation criteria, the number of treated patients among the study subjects, the duration of and type of psychotropic drug exposure, and diagnostic comorbidity—could conceivably lead to discrepant findings. The lack of significant group differences in caudate nucleus volume in our study is consistent with a meta-analysis of the functional neuroimaging literature that found similar caudate functioning in patients with OCD and in healthy comparison subjects after the results were normalized for global metabolism
(10). It is also possible that the use of other neuroimaging modalities may be required to detect caudate nucleus pathology in OCD. For example, in a spectroscopy study, Bartha et al.
(12) found decreased
N-acetylaspartate in the left corpus striatum of patients with OCD without associated group differences in volume, thus highlighting the purported sensitivity of this technique, compared to volumetry.
We did not replicate prior findings of smaller putamen volume in pediatric patients with OCD
(13), although the lack of group differences is consistent with a prior study of adult patients
(16). We considered whether operator drift could be one possible explanation; however, the average difference between putamen volumes measured twice for 16 subjects was less than 1%. It is possible that sampling differences between the present study and our prior study may at least partly account for the different findings. For example, 30% of the patients in the current study were male, compared to 68% of the patients in our prior study. The raw data from our prior study
(13) show that smaller putamen volume was most evident among male patients, relative to male comparison subjects, an effect that may have been undetected in the present study, given the fewer number of male patients. The patients in the present study were also more severely ill than the patients in our prior study, as reflected by the higher Children’s Yale-Brown Obsessive Compulsive Scale (mean=29 and mean=23, respectively) and Hamilton depression scale (mean=10 and mean=6, respectively) scores of the patients in the present study. These differences suggest that the patient groups in the two studies may not be directly comparable. Finally, methodologic differences in defining the putamen also may have contributed to the differences in findings.
In summary, we have reported the results of a structural MRI study in a new cohort of psychotropic drug-naive pediatric patients with OCD. The findings provide additional evidence for involvement of frontal and subcortical brain regions in the pathophysiology of OCD without the potentially confounding effects of exposure to psychotropic medication. Future studies with the goal of cross-validating the present findings by means of different research techniques, such as voxel-based morphometry, would be useful.