Glutamate’s role in schizophrenia has been investigated in recent years. Abnormal glutamatergic neurotransmission has been reported in animal schizophrenia models as well as in human postmortem and glutamate receptor antagonist studies (reviewed in reference
1) and implicated in the neuroarchitectural abnormalities documented in schizophrenia
(2). Glutamate abnormalities may also help explain the latency of expression of symptoms in schizophrenia within the context of the neurodevelopmental hypothesis for the illness
(3). Short-echo proton magnetic resonance spectroscopy (
1H-MRS) now allows us the opportunity to examine brain glutamate/glutamine in vivo.
The glutamate/glutamine system can only be examined reliably at higher magnetic field strengths (≥3 T) because there is multiple overlap of these resonances at field strengths <3 T. Most reliable studies report on glutamate/glutamine variables. (Only when one uses a field strength of >4.7 T are the glutamate and glutamine resonances completely resolved from one another.) A recent high-field strength study has reported increases in glutamine (suggesting greater than normal glutamatergic activity) in the anterior cingulate and thalamus in neuroleptic-naive first-episode patients relative to healthy comparison subjects
(4). What remains uncertain is the time course of glutamate/glutamine abnormalities prior to the onset of psychotic symptoms.
In this study, we investigated the glutamate/glutamine system in the medial prefrontal cortex of nonpsychotic adolescents at high genetic risk for schizophrenia. This region was chosen because it receives glutamatergic afferents from the thalamus as well as other cortical regions that have shown structural abnormalities in neuroimaging schizophrenia research
(5). We hypothesized that genetic liability would be expressed in glutamate/glutamine abnormalities in this cohort and be correlated with functional assessments.
Method
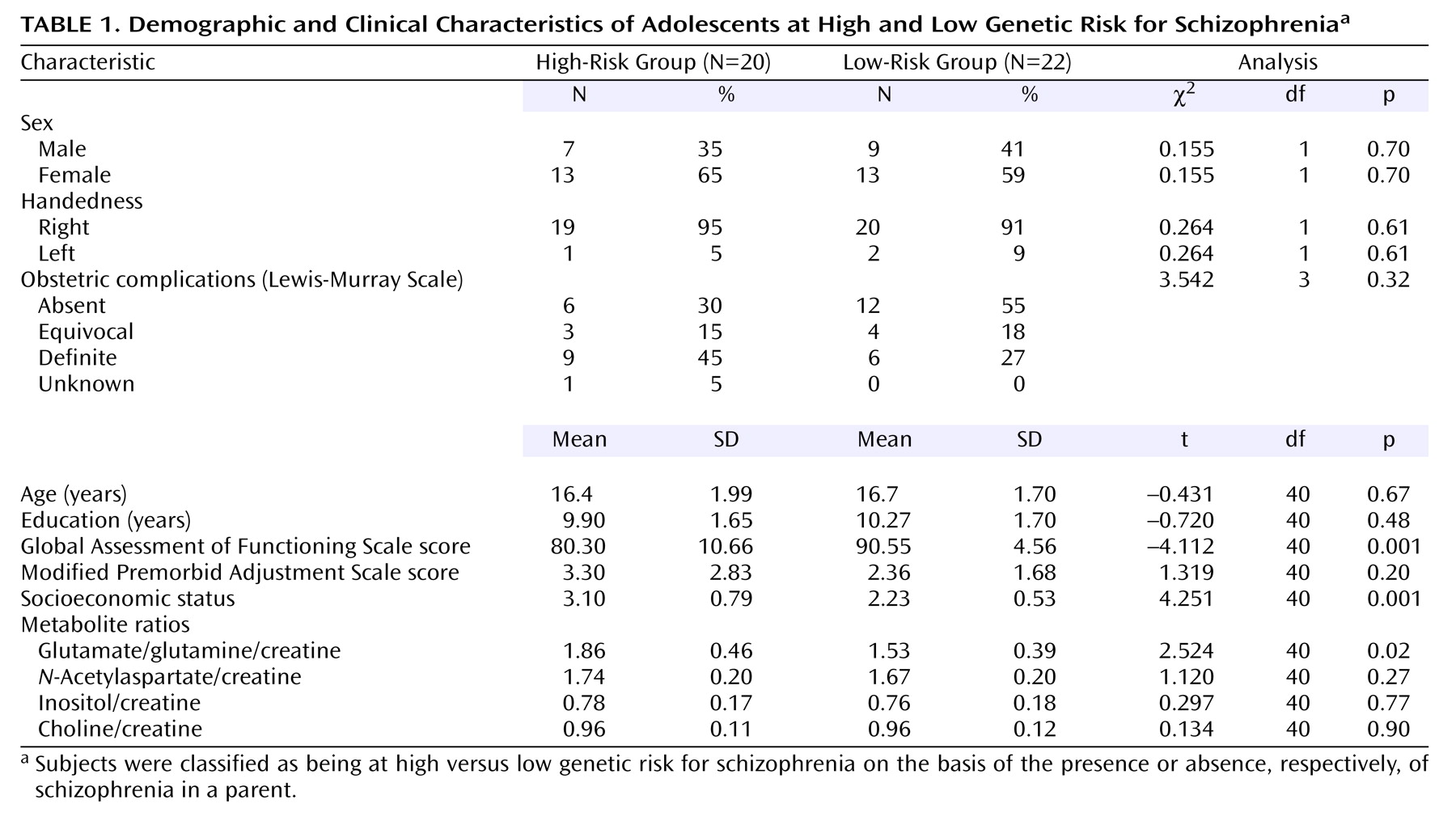
After receiving informed consent from parents to approach their child (schizophrenia parents were recruited through the local university schizophrenia clinic), informed consent was then obtained from 20 asymptomatic adolescents at high genetic risk for schizophrenia (i.e., those who had a parent with schizophrenia) and a comparison group of 22 adolescents at low genetic risk (i.e., no parent with a history of schizophrenia). All subjects were administered the Diagnostic Interview for Children and Adolescents or the Structured Clinical Interview for DSM-IV, depending on age, to rule out psychopathology. Other exclusion criteria were major neurological/medical illness, substance/alcohol abuse, and history of significant head injury (loss of consciousness >20 minutes). Overall functioning was assessed with the Global Assessment of Functioning Scale (GAF), parental socioeconomic status with the Hollingshead scale, history of obstetric complications with the Lewis-Murray scale, and psychosocial adjustment with the Modified Premorbid Adjustment Scale.
1H-MRS was performed using a 3-Tesla magnet (Magnex Scientific, Concord, Calif.) equipped with actively shielded gradients and a spectrometer (Surrey Medical Imaging Systems, Surrey, U.K.) equipped with a quadrature birdcage resonator. Transverse, sagittal, and coronal gradient echo images (TE=20 msec, TR=500 msec, 5 mm slice thickness, 256×256 point resolution) were acquired to register a 2.5-cm3 volume of interest in the right medial frontal cortices. In the transverse and coronal planes, the anterior/posterior or superior/inferior edge of the voxel was rotated to be parallel to the brain midline, and the inner edge of the voxel was located 4 mm to the right of the midline. In the sagittal images, the lower edge of the voxel was rotated to be at the same level as a line extending through the anterior commissure-posterior commissure line, while the posterior edge was positioned so that it was adjacent to the most superior aspect of the corpus callosum. This voxel placement maintained a consistent mix of gray matter to white matter to CSF in the subjects.
Shimming to less than 0.05 ppm was accomplished by using both FASTMAP
(6) and an “in-house” auto shim routine (for fine adjustment of linear shim currents). Water-suppressed STEAM localized spectra were acquired (TR=3 seconds, TE=20 msec, and TM=30 msec; TI was approximately 600 msec) and were the sum of 256 averages, acquired in 32 blocks of eight averages. This allowed each of the 32 subspectra to be examined for spectral artifacts due to subject movement or hardware fluctuations prior to their final summing. Furthermore, it allowed us where necessary to re-register each of the 32 subspectra to the same frequency reference, again prior to summing. This eliminated any apparent frequency drift that would broaden the spectral lines and degrade the quality of the summed spectrum.
The MRS raw data were analyzed by using the LCModel analysis program
(7). Prior to this analysis, a preliminary inspection of the data was performed following Fourier transformation of the 32 subspectra. Zero- and first-order phase corrections were applied to each subspectrum, then the frequency of the
N-acetylaspartate-methyl peak was determined in each to allow precise frequency registration. Following frequency registration, the subspectra were added and an inverse Fourier transformation was performed to generate the time domain real/imaginary pair suitable for importing into the analysis program. This preparation of the data prior to final analysis was performed in the MATLAB program environment. Numerically simulated time domain data
(8) were used as basis spectra for the brain metabolites accounted for in the LCModel program. Recently published metabolite chemical shifts and coupling constants
(9) were used in the numerical simulations.
Results
The demographic, clinical, and metabolite variables studied are presented in
Table 1. The groups were significantly different in terms of global functioning, socioeconomic status, and glutamate/glutamine. For all subjects, glutamate/glutamine was not correlated with age (r=–0.07, df=40, p=0.66), socioeconomic status (r=0.05, df=40, p=0.73), or GAF score (r=0.09, df=40, p=0.59). However, since socioeconomic status and GAF scores significantly differed between groups, correlations were also performed per group. Glutamate/glutamine was correlated only with GAF score in the high-risk group (r=0.57, df=18, p=0.009).
Discussion
In this study we report, to our knowledge, the first short-echo high-field
1H-MRS study in asymptomatic high-risk adolescents focusing on glutamate/glutamine. A previous low-field
1H-MRS study that had a similar cohort (but a smaller sample size) reported a trend for decreased
N-acetylaspartate/choline ratios in the anterior cingulate region but did not investigate glutamate/glutamine
(10).
The neurodevelopmental model of schizophrenia was strengthened with the demonstration of neurobehavioral and cognitive abnormalities in individuals who, at the time of investigation, demonstrated no psychopathology but later went on to develop schizophrenia
(11). It is hypothesized that some of the significant aspects of the premorbid substrate of schizophrenia are formed before the onset of the illness. Our findings of glutamate/glutamine abnormalities in medial frontal cortices of a genetic high-risk group lends support for the neurodevelopmental hypothesis and is complementary to the recent findings of increased glutamate/glutamine in a first-episode group.
Glutamate system dysfunction may play a role in neuroarchitectural abnormalities seen in schizophrenia
(3), and higher than normal glutamatergic metabolites at the early stages of the illness may lead to excitotoxicity, resulting in observed decreased
1H-MRS metabolites in chronic schizophrenia. With respect to our study, follow-up of these adolescents through early adulthood would clarify if this glutamate/glutamine abnormality is an indicator of genetic liability or a clue to who, among unaffected relatives of individuals with schizophrenia, may be at an increased risk for the illness.