The middle temporal gyrus is located on the lateral surface of the temporal lobe ventral to the superior temporal gyrus. The inferior temporal gyrus is located on the lateral and inferior surfaces of the temporal lobe, ventral to the middle temporal gyrus. Previous functional neuroimaging studies have suggested that the middle temporal gyrus and inferior temporal gyrus are involved in several cognitive processes
(1), including language and semantic memory processing (middle temporal gyrus)
(1–
3), as well as visual perception (inferior temporal gyrus)
(4,
5) and multimodal sensory integration
(6). Functional deficits in these cognitive domains, i.e., language
(7), semantic memory
(8), and complex visual perception
(9), have been reported in schizophrenia. Thus, it may be reasonable to assume that the middle temporal gyrus and inferior temporal gyrus play important roles in the pathophysiology of schizophrenia. However, these regions have received little attention in structural magnetic resonance imaging (MRI) studies of schizophrenia.
For temporal lobe cortical structures, left superior temporal gyrus gray matter abnormality is the most frequently reported finding in patients with schizophrenia
(10). The superior temporal gyrus includes the primary and secondary auditory cortices and a language-related area. Barta et al.
(11) reported that volume reduction in the left anterior region was associated with hallucinations, and Shenton et al.
(12) reported that volume reduction in the left posterior superior temporal gyrus was associated with thought disorder.
Subsequent reports have suggested that the left middle temporal gyrus is associated with hallucinations. McGuire et al.
(13) suggested that a predisposition to auditory verbal hallucinations in schizophrenia was associated with a failure to activate the left middle temporal gyrus and the rostral supplementary motor area thought to be involved with the monitoring of inner speech. Additionally, several other functional MRI (fMRI) studies have reported that increased activation in the right middle temporal gyrus was associated with auditory verbal hallucinations in schizophrenia
(14–
16). Lennox et al.
(15) interpreted the finding of decreased left hemisphere activation and increased right hemisphere activation in the middle temporal gyrus during auditory hallucinations as possibly reflecting abnormal right hemisphere lateralization of language in schizophrenia. With respect to functional correlates of the inferior temporal gyrus, a positron emission tomography study
(17) showed a low glucose metabolic rate in the inferior temporal gyrus of schizophrenia patients during a continuous performance test. In this study, the investigators reported that patients had lower metabolism than healthy subjects, a finding that was significantly associated with poorer test performances, although the exact role of the inferior temporal gyrus in the continuous performance test was unknown.
Despite these functional correlates, few structural MRI studies in schizophrenia have examined as anatomic regions of interest the middle temporal gyrus and inferior temporal gyrus. Using a semiautomated gyral identification program, Goldstein et al.
(18) found no difference in middle and inferior temporal gyrus gray matter volumes between chronic schizophrenia and normal subjects. Downhill et al.
(19) divided the temporal lobe into superior temporal gyrus and nonsuperior temporal gyrus gray matter regions, without specifically delineating the middle temporal gyrus and inferior temporal gyrus. They reported smaller whole temporal lobe gray matter volume in patients with chronic schizophrenia, with volume reduction more marked for nonsuperior temporal gyrus volumes. Highley et al.
(20) measured temporal pole, superior temporal gyrus, and middle and inferior temporal gyrus volumes in a postmortem study and found bilateral gray matter volume reductions in the anterior inferior temporal gyrus of schizophrenia brains that approached significance relative to matched comparison subjects. Using voxel-based morphometry, Job et al.
(21) reported significant gray matter reductions in the left middle temporal gyrus, left postcentral gyrus, left limbic lobe, right anterior cingulate, and the right medial frontal lobe in patients with first-episode schizophrenia. However, Kubicki et al.
(22) did not find voxel-based morphometry reductions in these regions in first-episode patients.
In the current study, middle and inferior temporal gyrus gray matter volumes were measured in chronic schizophrenia patients and normal comparison subjects with high-spatial-resolution MRI (0.9375-mm
3 voxels in resampled slices) with three-dimensional information to provide more reliable measurement of these brain regions. For comparative purposes we also computed superior temporal gyrus and fusiform gyrus gray matter volumes in the present sample. For each temporal lobe gyrus, we investigated the association between its gray matter volume and clinical symptoms as measured with the Scale for the Assessment of Positive Symptoms (SAPS)
(23) and the Scale for the Assessment of Negative Symptoms (SANS)
(24). We predicted that in this group of patients with chronic schizophrenia, gray matter volumes of the left superior temporal gyrus or middle temporal gyrus would be negatively correlated with severity of hallucinations.
Method
Subjects
Twenty-three male patients with chronic schizophrenia and 28 healthy male comparison subjects participated in this study. Patients were recruited from the VA Boston Healthcare System–Brockton Division. The comparison subjects were recruited through newspaper advertisement. Subjects in the present study included two new subjects and 49 subjects (21 patients and 28 comparison subjects) common to our most recently published region of interest study of the fusiform gyrus in chronic schizophrenia
(25). None of the patients or comparison subjects was common to our earlier superior temporal gyrus study in chronic schizophrenia
(12). After a complete description of the study, written informed consent was obtained from all participants.
Demographic data for subjects in each group are presented in
Table 1. Exclusion criteria were 1) neurologic illness or major head trauma, 2) previous treatment with ECT, 3) alcohol or drug dependence, or 4) alcohol or drug abuse within the past 5 years. The age range for inclusion was 20 to 55 years. Comparison subjects were screened with the Structured Clinical Interview for DSM-III-R (SCID), nonpatient edition, by trained interviewers (M.F., M.E.S.). No comparison subject had an axis I psychiatric disorder or a first-degree relative with an axis I psychiatric disorder.
All patients were diagnosed with schizophrenia per DSM-III-R criteria, using information from the SCID by the same trained interviewers (M.F., M.E.S.). All patients were receiving neuroleptic medication (typical, N=10; atypical, N=12; both, N=1). The mean dose was 504 mg/day (SD=268) in chlorpromazine equivalents.
Clinical Evaluations
Handedness was assessed with the Edinburgh Inventory
(26). Socioeconomic status of subjects and parental socioeconomic status were measured by the Hollingshead two-factor index (1=highest, 5=lowest). All subjects were given the WAIS-R information subscale as an estimate of gross fund of information. In the present study, we investigated correlations between regions of interest and clinical symptoms as measured by the SAPS and the SANS (
Table 1). According to clinical interviews, the SAPS, and medical records, more than one-half of the patients had auditory hallucinations (N=13). The remainder had somatic hallucinations (N=1), hallucinations with unspecified modality (N=1), no definite information regarding hallucinations (N=2), or no hallucinations (N=6).
MRI Image Acquisition and Processing
MR images were acquired with a 1.5-T General Electric scanner (GE Medical Systems, Milwaukee) at the Brigham and Women’s Hospital in Boston. Imaging methods have been described in detail elsewhere
(27). The acquisition protocol included two MRI pulse sequences. The first sequence resulted in contiguous spoiled gradient-recalled images (repetition time=35 msec, echo time=5 msec, one repetition, 45° nutation angle, 24-cm field of view, number of excitations=1, matrix=256×256 [192 phase-encoding steps]×124). Voxels were 0.9375×0.9375×1.5 mm. Data were formatted in the coronal plane and analyzed as 124 coronal 1.5-mm-thick slices. The second acquisition sequence resulted in an axial series of contiguous double-echo (proton density and T
2-weighted) images (repetition time=3000 msec, echo time=30 and 80 msec, 24-cm field of view, and an interleaved acquisition with 3.0-mm slice thickness). The voxel dimensions were 0.9375×0.9375×3.0 mm. This latter pulse sequence was used to measure the volume of the total intracranial contents (brain, CSF, connective tissue, and blood vessels). An anisotropic diffusion filter
(28) was applied to both spoiled gradient-recalled and T
2 images to reduce noise prior to processing. The intensity information from both the spoiled gradient-recalled and T
2 images was used in a fully automated segmentation program to classify tissue into gray matter, white matter, and CSF. An iterative expectation-maximization algorithm estimated image intensity inhomogeneities, applied intensity corrections on the basis of these estimates, and then classified tissue on the basis of the same set of signal intensity parameters for all subjects
(29). Images were realigned by using the line between the anterior and posterior commissures and the sagittal sulcus to correct head tilt and then were resampled into isotropic voxels (0.9375 mm
3). Manual drawings of regions of interest were performed on the realigned and resampled coronal slices.
Regions of Interest
The detailed guidelines of Kim et al.
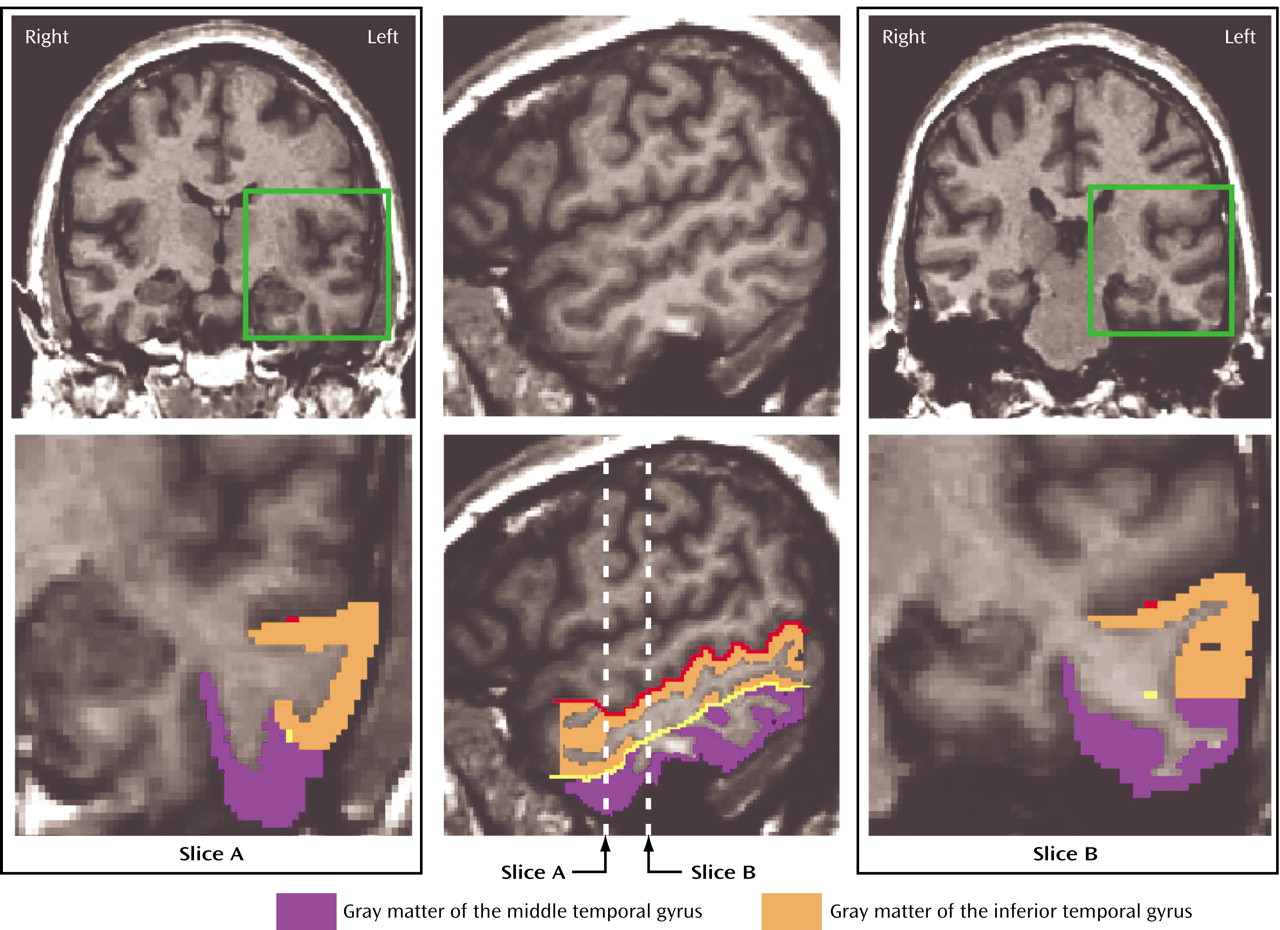
(30) were used for a topographic segmentation method for the inferior temporal gyrus and middle temporal gyrus. First, the rater traced the superior temporal sulcus and inferior temporal sulcus on sagittal images. When the superior temporal sulcus and inferior temporal sulcus were interrupted, guidelines were drawn extending the sulcal course across the tissue bridge of white matter. These two lines were seen as points on each coronal image, which served as a guide for the separation of middle temporal gyrus and inferior temporal gyrus (
Figure 1). In the 17 cases with a fragmented inferior temporal sulcus (five interruptions), the course of the superior temporal sulcus and inferior temporal sulcus was defined by consensus of judges (T.O., C.C.D., M.E.S., R.W.M.), guided by the atlas of Ono et al.
(31).
Middle temporal gyrus
Manual drawings of the middle temporal gyrus were performed on the coronal plane (slice A in
Figure 1). Superior temporal sulcus or anterior occipital sulcus was used as the superior border of the middle temporal gyrus, and the inferior temporal sulcus served as the inferior border. On a slice where there was a transitional area between the middle temporal gyrus and inferior temporal gyrus, the middle temporal gyrus and inferior temporal gyrus were divided operationally by extending the guideline horizontally to the lateral surface of the temporal lobe (slice B in
Figure 1). The anterior boundary was defined as the first slice containing the intact temporal stem. The anterior tip of the parietooccipital sulcus, as identified on the midsagittal plane, determined the posterior boundary of the middle temporal gyrus.
Inferior temporal gyrus
The inferior temporal sulcus was used as the superior border of the inferior temporal gyrus, and the occipitotemporal sulcus was used as the inferior border. This sulcus is interrupted frequently
(31). In such interrupted cases, the border was decided as the more prominent sulcus on the coronal and axial slices. The anterior and posterior boundaries were the same as for the middle temporal gyrus.
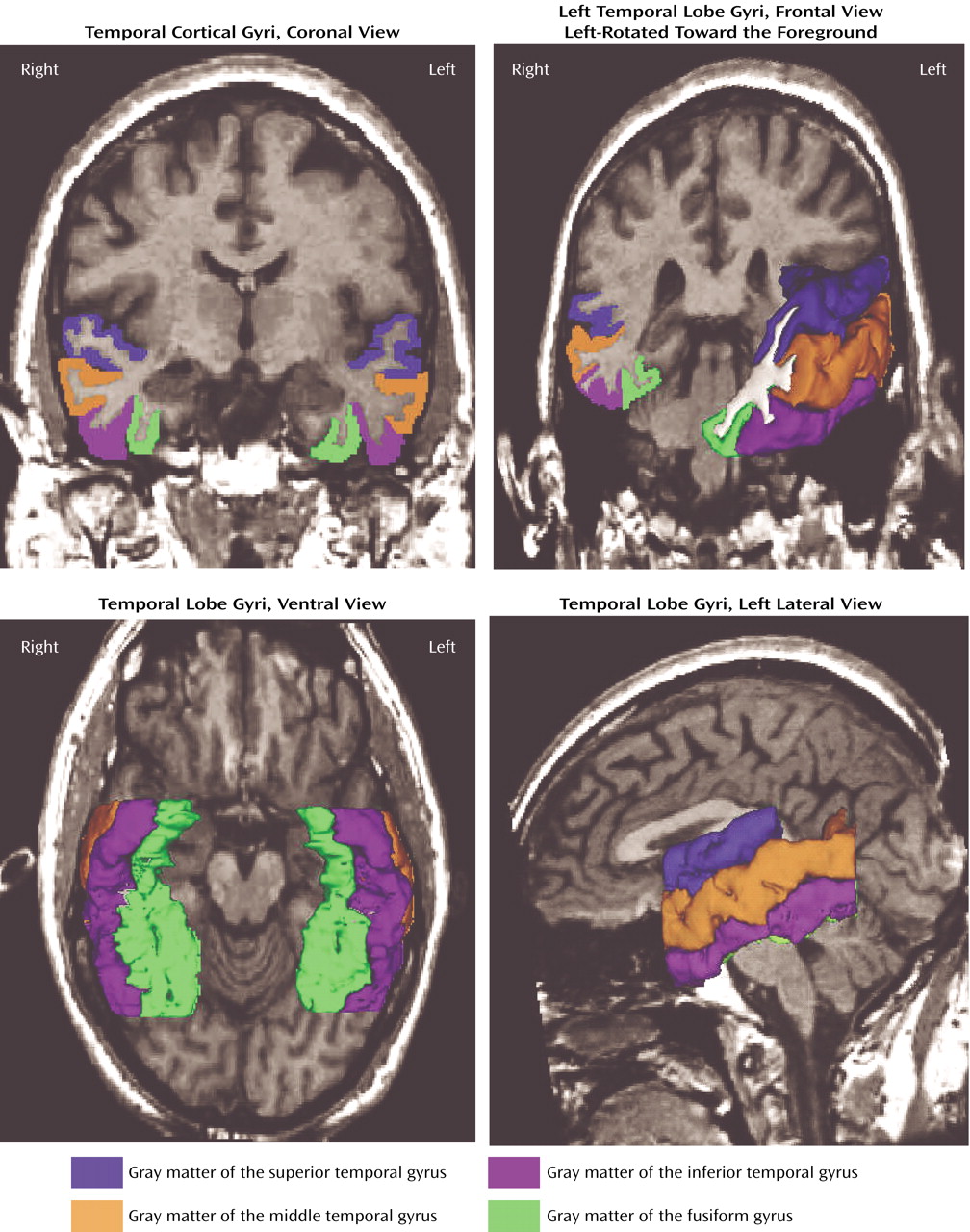
Figure 2 shows the superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, and fusiform gyrus in a coronal slice and three-dimensional reconstructions.
Interrater reliability was computed for the regions of interest by three independent raters (T.O., K.K., and S.K.T.) blind to group membership. We randomly selected six cases, all with a fragmented superior temporal sulcus or inferior temporal sulcus. The raters first drew guidelines and then measured middle and inferior temporal gyri on every third slice. The intraclass correlations for interrater reliability were high: 0.98 for left middle temporal gyrus, 0.98 for right middle temporal gyrus, 0.96 for left inferior temporal gyrus, and 0.97 for right inferior temporal gyrus.
Superior temporal gyrus and fusiform gyrus
Definition of the superior temporal gyrus and fusiform gyrus followed that of our previous publications
(25,
32). Briefly, for the superior temporal gyrus, the anterior boundary was defined as the first slice containing the intact temporal stem. The posterior landmark was determined by the last slice that included the crux of the fornix. The superior temporal sulcus was used as the inferior border. For the fusiform gyrus, the anterior landmark was defined by one slice posterior to the mamillary body. The posterior landmark was determined by the anterior tip of the parieto-occipital sulcus in the midsagittal plane. The collateral sulcus was used as the medial border. The occipitotemporal sulcus was used to determine the lateral border.
Statistical Analyses
We used t tests to assess group differences in age, handedness, WAIS-R information subscale scores, socioeconomic status, parental socioeconomic status, and total intracranial contents. Group differences in regions of interest were evaluated by using relative volumes ([absolute region of interest volume]/[intracranial contents] × 100); for comparative purposes, absolute volumes are also presented. Of note, the statistical conclusions were the same when the intracranial contents minus gray matter volume of the whole temporal lobe neocortex (superior temporal gyrus plus middle temporal gyrus plus inferior temporal gyrus plus fusiform gyrus) was used for the correction instead of the intracranial contents. In addition, the statistical conclusions that will be subsequently reported remained the same when absolute volume was analyzed with the intracranial contents as a covariate. For relative volume comparisons, a mixed-model repeated-measures analysis of variance (ANOVA) was performed separately for each gyrus with group (schizophrenia, comparison) as a between-subject factor and hemisphere (left, right) as a within-subject factor.
To investigate the laterality of volume abnormalities in the temporal lobe gyri, we compared the relative volumes of the superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, and fusiform gyrus in a pairwise manner for similarities and differences in asymmetries in the current 23 subjects with chronic schizophrenia. Repeated-measures ANOVA was performed with gyrus (each gyrus in the pair being compared) and hemisphere (left or right) as within-subject factors. Finally, Spearman’s correlation evaluated the correlations between clinical symptom scales and absolute gray matter volumes of the temporal lobe gyri (in our data absolute MRI volumes have yielded the most accurate correlational structure [
12,
25,
33]). In this analysis we used p<0.017 as the cutoff value for statistical significance for each gyrus and each of the three clinical symptom measures (SAPS total score, SANS total score, and the SAPS hallucination subscale score).
Results
There were no significant group differences in age, handedness, parental socioeconomic status, or WAIS-R information (
Table 1). Patients showed lower socioeconomic status than comparison subjects, which is consistent with reduced functioning due to the disorder.
Gray Matter Volume of Temporal Lobe Regions
Middle temporal gyrus
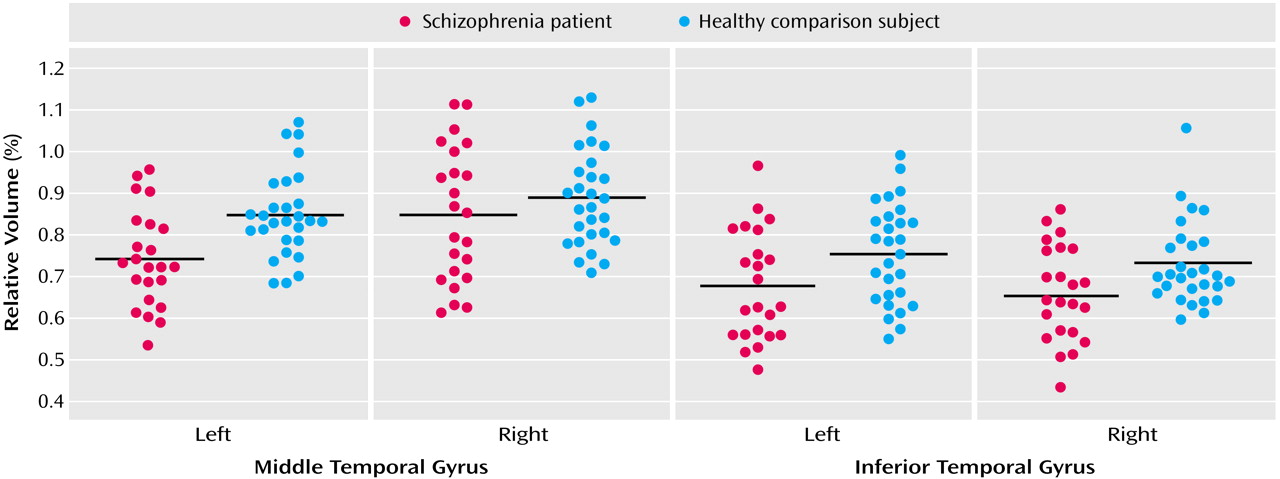
Relative middle temporal gyrus volumes were significantly smaller in the schizophrenia patients (F=5.22, df=1, 49, p<0.03) (
Table 2 and
Figure 3). The right middle temporal gyrus was larger than the left (F=23.54, df=1, 49, p<0.001), and there was a significant group-by-hemisphere interaction (F=5.74, df=1, 49, p=0.02). We then evaluated each group for middle temporal gyrus hemispheric asymmetry. Significantly greater right versus left gray matter volume in the middle temporal gyrus was seen in both the healthy subjects (t=2.09, df=27, p<0.05) and schizophrenia patients (t=4.51, df=22, p<0.001). To further delineate the source of the group-by-hemisphere interaction in middle temporal gyrus volumes, we compared groups for each hemisphere. As seen in
Table 2, there was no significant between-group difference for the right hemisphere; for the left hemisphere, a significant reduction (12%) in middle temporal gyrus gray matter volume was seen in the schizophrenia patients relative to the healthy subjects.
Inferior temporal gyrus
A repeated-measures ANOVA revealed a significant main effect of group (F=7.05, df=1, 49, p<0.02), with no significant group-by-hemisphere interaction (F=0.06, df=1, 49, p=0.80). These results indicated that schizophrenia patients had significant bilateral reductions (10.4% on the left and 9.6% on the right) in inferior temporal gyrus gray matter volume relative to comparison subjects (
Table 2).
Superior temporal gyrus and fusiform gyrus
For the superior temporal gyrus, a repeated-measures ANOVA showed a significant group-by-hemisphere interaction (F=16.00, df=1, 49, p<0.001), indicating left superior temporal gyrus reduction in patients. For the fusiform gyrus, a repeated-measures ANOVA showed a significant main effect of group (F=7.09, df=1, 49, p=0.01), with no group-by-hemisphere interaction (F=0.94, df=1, 49, p=0.34), indicating bilateral fusiform gyrus reduction in patients.
Laterality of Temporal Lobe Gray Matter Volume Abnormalities in Schizophrenia
Superior temporal gyrus and middle temporal gyrus were more reduced in the left hemisphere than in the right (main effect of hemisphere: F=52.08, df=1, 22, p<0.001), and the degree of left-less-than-right asymmetry did not differ between the superior temporal gyrus and middle temporal gyrus (gyrus-by-hemisphere interaction: F=0.81, df=1, 22, p=0.38). Gyrus-by-hemisphere interactions showed significantly more left hemisphere reduction relative to that seen in the inferior temporal gyrus for both the superior temporal gyrus (F=22.2, df=1, 22, p<0.001) and middle temporal gyrus (F=8.29, df=1, 22, p=0.009). Similarly, gyrus-by-hemisphere interactions showed significantly more left hemisphere reduction relative to that seen in the fusiform gyrus for both the superior temporal gyrus (F=47.82, df=1, 22, p<0.001) and middle temporal gyrus (F=16.00, df=1, 22, p=0.001). Inferior temporal gyrus and fusiform gyrus did not show differences in asymmetrical reductions (main effect of hemisphere: F=0.21, df=1, 22, p=0.089, gyrus-by-hemisphere interaction: F=1.37, df=1, 22, p=0.25).
Correlations Between Region of Interest Volumes and Clinical Measures
Age, socioeconomic status, parental socioeconomic status, duration of illness, and neuroleptic dose were not significantly correlated with any region of interest volumes in patients with schizophrenia.
For the correlations between absolute gray matter volumes of the temporal lobe gyri and total clinical symptom scales, there was a significant negative correlation between total scores on the SAPS and left superior temporal gyrus volume (rs=–0.58, p=0.005) but no significant correlations with the other gyri nor any significant correlation between gyral volume and SANS score. As predicted, the score on the global rating of hallucinations on the SAPS was negatively correlated with both the left superior temporal gyrus volume (rs=–0.79, p<0.001) and left middle temporal gyrus volume (rs=–0.61, p=0.004), but not with the other gyral gray matter volumes.
Since the score on hallucinations was significantly negatively correlated with the left superior temporal gyrus and middle temporal gyrus volumes, t tests were used for additional (exploratory) analyses of volume differences between the 13 patients with auditory hallucinations and the six patients without. The patients with auditory hallucinations had significantly smaller left gray matter volumes than did those without in both the superior temporal gyrus (t=–5.90, df=17, p<0.001) and middle temporal gyrus (t=–2.22, df=17, p=0.04), whereas there were no significant differences on the right (superior temporal gyrus: t=–1.96, df=17, p=0.07; middle temporal gyrus: t=–1.53, df=17, p=0.15).
Discussion
High-resolution MRI, three-dimensional information, and neuroanatomically based, reliable boundary definitions were used to investigate gray matter volumes of the middle temporal gyrus and inferior temporal gyrus. To our knowledge, this is the first study to demonstrate gray matter volume reductions in the left middle temporal gyrus and bilateral inferior temporal gyrus in patients with chronic schizophrenia. Moreover, patients with schizophrenia showed a left-lateralized pattern of dorsal (superior temporal gyrus and middle temporal gyrus) temporal lobe gray matter reductions, whereas ventral differences (inferior temporal gyrus and fusiform gyrus) were bilateral.
To our knowledge, there have been no previous studies of the middle temporal gyrus and inferior temporal gyrus in schizophrenia that have used anatomically defined regions of interest, and only a small number of MRI volumetric studies of any kind. Differences in region of interest definition and methodology may account for the reported discrepancies. The postmortem study by Highley et al.
(20) did not use the entire extent of the middle temporal gyrus and inferior temporal gyrus. Moreover, it remains an open question how closely the semiautomated cortical parcellation method
(18) and the voxel-based morphometry methods
(21,
22) parallel manually drawn and anatomic landmark-based regions of interest. Our recent voxel-based morphometry versus manual region-of-interest comparison in the same group of patients with first-episode schizophrenia
(22) found only partial congruence.
With respect to clinical correlations, increased severity of global hallucinatory experiences, mostly auditory, was significantly associated with smaller left superior temporal gyrus and middle temporal gyrus volumes. Patients with auditory hallucinations had significantly smaller superior temporal gyrus/middle temporal gyrus volumes on the left than those without this symptom. These data were highly consistent with a previous study of the superior temporal gyrus
(11) and a study of the middle temporal gyrus in which patients with schizophrenia who were predisposed to auditory hallucinations showed reduced activation of the left middle temporal gyrus when imagining sentences in another person’s voice
(13). Several other fMRI studies have reported decreased left and increased right middle temporal gyrus activation in schizophrenia during auditory verbal hallucinations
(14–
16). The association of hallucinations, mainly auditory, with volumetric abnormalities of the left superior temporal gyrus and middle temporal gyrus in the present study points to a possible anatomical substrate. Conversely, the absence of correlations with SANS scores suggests these regions may not be prominently associated with negative symptoms.
With respect to laterality of temporal lobe abnormalities in schizophrenia, both the superior temporal gyrus and middle temporal gyrus were significantly smaller in the left hemisphere than the inferior temporal gyrus and fusiform gyrus, which showed bilateral volume reductions. The pattern thus emerges of dorsal temporal gyri (superior temporal gyrus, middle temporal gyrus) showing left-lateralized smaller volumes, and the ventral gyri (inferior temporal gyrus and fusiform gyrus) showing bilateral smaller volumes. This dorsal lateralization may be related to the left-lateralization of language, since functional neuroimaging studies of language have shown predominantly left-lateralized activation of the superior temporal gyrus and middle temporal gyrus
(1), and left-lateralized functional dominance for language is a feature in individuals who are right-handed
(34), as were the subjects in the present study. In contrast, there is no clear evidence for hemispheric dominance in visual processing, a function which likely involves the inferior temporal gyrus and fusiform gyrus.
There are several limitations of this study. First, the present study cannot answer the question of whether the volume reduction was progressive in the peri- or postonset course of the illness or the extent to which it was developmental. It will thus be important to investigate whether middle temporal gyrus/inferior temporal gyrus gray matter volume undergoes a progressive decrease over the 1.5 years following first hospitalization, as we have found for the superior temporal gyrus
(32). Second, the present study also did not allow us to exclude the effect of chronic treatment with neuroleptic medication on middle temporal gyrus/inferior temporal gyrus gray matter abnormalities in patients (although no volume measure correlated with neuroleptic dosage), nor demonstrate the specificity to schizophrenia psychosis. It will thus be important to investigate whether middle and inferior temporal gyrus abnormalities are observed or not in patients with schizophrenia and affective psychosis at their first hospitalization, with minimal or no medication history. Third, this study does not provide the answer of whether or not female patients show gray matter volume abnormalities of the middle temporal gyrus/inferior temporal gyrus, since this study did not include any female subjects. Fourth, in the current study, only gray matter but not white matter volumes for the superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, and fusiform gyrus were investigated.
In conclusion, we wish to underline the usefulness of chronic schizophrenia for establishing correlations between anatomic regions of interest and clinical symptoms and signs. At least in our hands, clinical correlations have been more clearly revealed in chronic than in first-episode schizophrenia, perhaps because of the greater symptom/sign stability in chronic schizophrenia (see, for example, references
12,
25, and
27 as well as the findings of the present study).
In summary, the present study chose consistent neuroanatomical boundaries for defining the middle temporal gyrus and inferior temporal gyrus, using three-dimensional information. There were smaller gray matter volumes in the left middle temporal gyrus and bilateral reductions in the inferior temporal gyrus in patients with schizophrenia relative to normal comparison subjects. In addition, more severe hallucinations were significantly correlated with smaller left superior temporal gyrus and middle temporal gyrus volumes. In conjunction with findings of reductions in the left superior temporal gyrus and bilateral fusiform gyrus, these data suggest that schizophrenia may be characterized by left hemisphere-selective dorsal pathophysiology and bilateral ventral pathophysiology in temporal lobe gray matter.