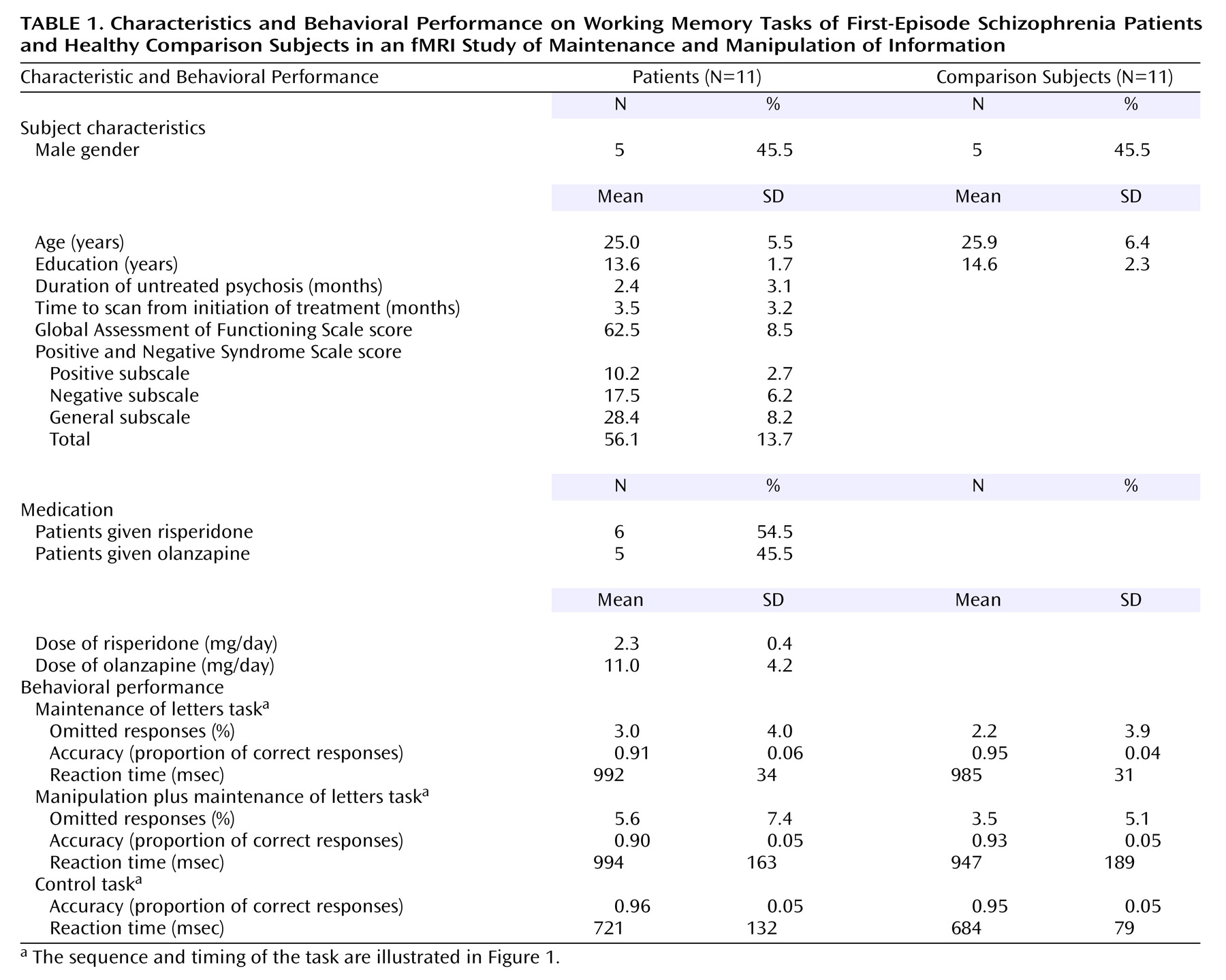
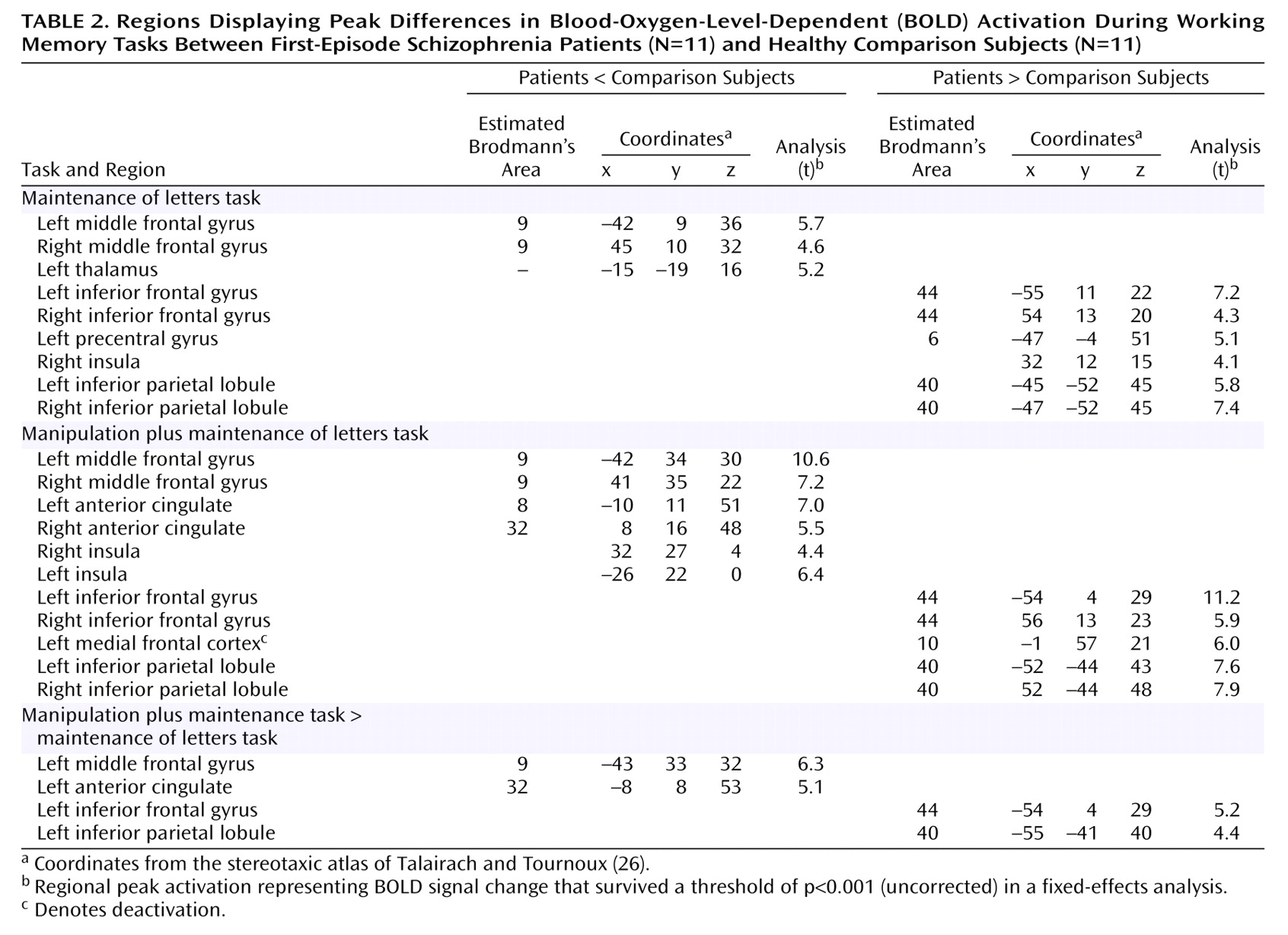
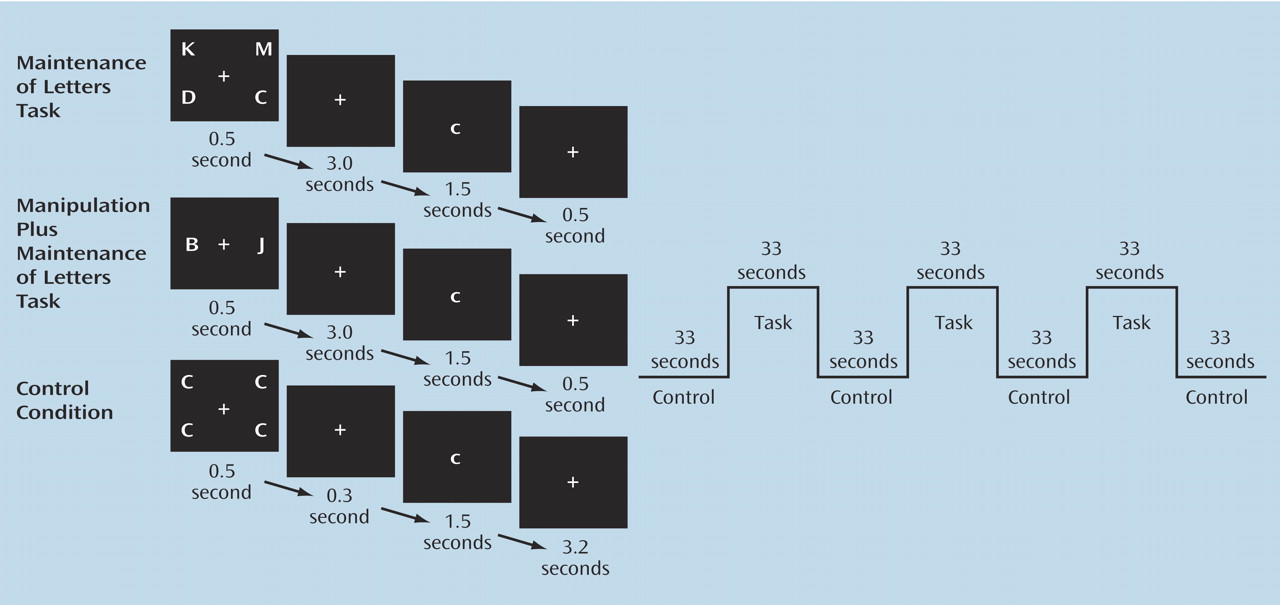
Working memory is a limited-capacity system that enables maintenance and manipulation of information temporarily. It plays an important role in higher-order thinking, language, and goal-directed behavior
(1), and it is an important facet of the cognitive dysfunction in schizophrenia
(2,
3). A number of neuroimaging studies have sought to uncover the neural basis of working memory deficits in patients with schizophrenia. Reduced dorsolateral prefrontal cortex (i.e., Brodmann’s area 46, 9) activation
(4,
5), increased activation
(6), an absence of differences in activation
(7), and a combination of increased and decreased activation
(8) have all been described. The anatomically and functionally related ventrolateral prefrontal cortex (Brodmann’s area 44, 45, 47) has been reported to show decreased activation in some studies
(9) but not in others
(4,
8).
These divergent findings could arise from non-disease-related factors, for example, task performance
(6,
10), working memory load
(6,
8), and the cognitive paradigm used in the study
(11). Many previous experiments evaluating working memory engaged the various components of this faculty but did not explicitly attempt to dissociate the effects of the illness on these components. An important goal of the present study was to explore this possibility in terms of the effect on prefrontal activation of the interaction of task component and disease. Illness-related factors such as duration of illness, occupational and social deprivation, and substance abuse are thought to affect brain anatomy and function
(12) and may be a further confounder in the effort to define disease-specific working memory dysfunction. Thus, only first-episode schizophrenia patients were evaluated in our study.
One approach that has pointed to specific dorsolateral prefrontal cortex dysfunction is isolation of context processing in working memory
(4). Another approach, the one we used in this study, is to consider the maintenance and manipulation processes separately. For a given load, the addition of explicit manipulation requirements results in increased activation, particularly in the dorsolateral prefrontal cortex, in normal individuals
(13,
14). (Here, the term “load” refers to the number of items that have to be maintained in working memory [e.g., in a Sternberg-type maintenance task]. “Task complexity” refers to the number of cognitive processes required to perform a task. “Task difficulty” is dependent on one or both of these conditions; when comparing tasks tapping different domains, as in the current study, reaction time and accuracy are used to gauge difficulty.) The dorsolateral prefrontal cortex also has a role in the maintenance of information
(15,
16), whereas the left ventrolateral prefrontal cortex and left inferior parietal lobule are thought to participate in rehearsal processes that briefly maintain verbal information
(17,
18).
In this study, we used two working memory tasks that evaluated prefrontal function associated with maintenance alone and with manipulation in addition to maintenance
(19). We tested working memory using a two-by-two experimental design, with the expectation that prefrontal activation in the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex would diverge more prominently in patients, relative to comparison subjects, particularly in the manipulation plus maintenance task, as suggested by recent behavioral experiments
(20).
Discussion
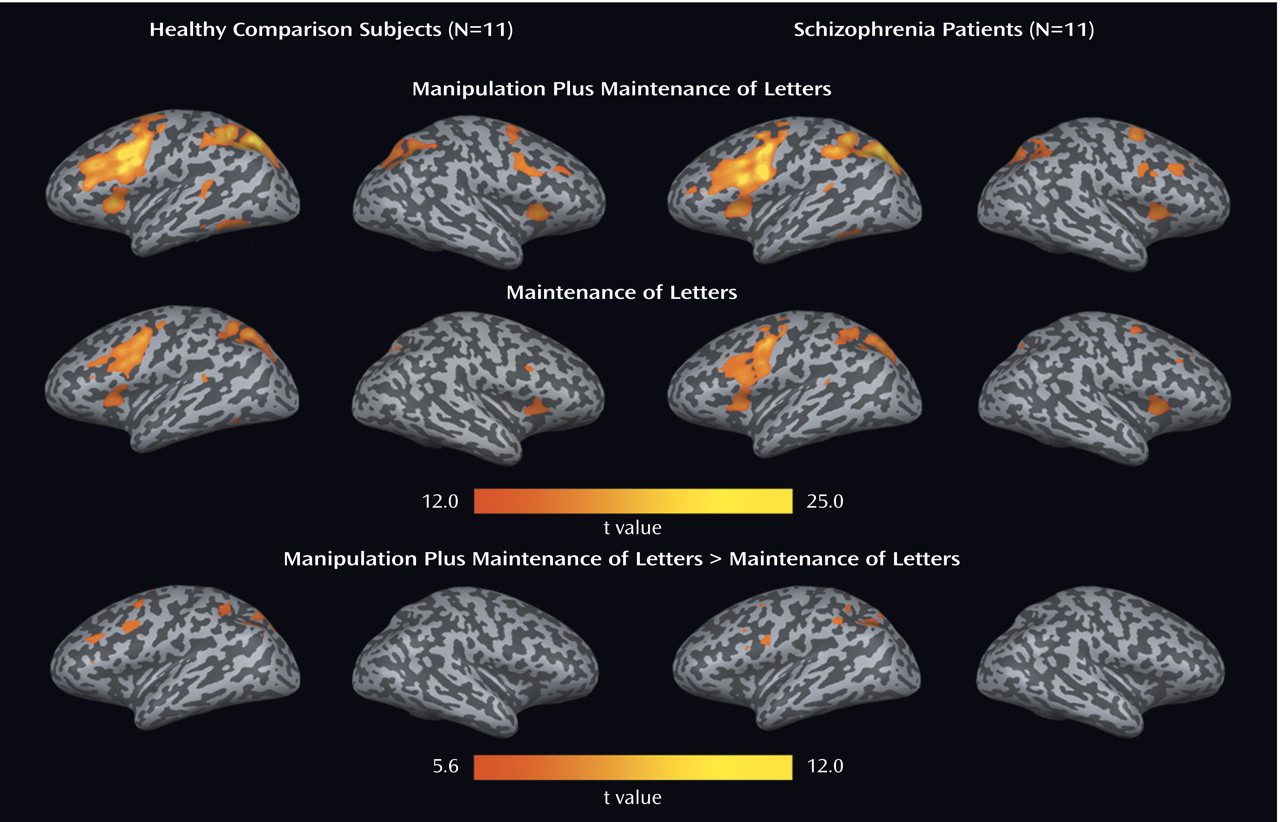
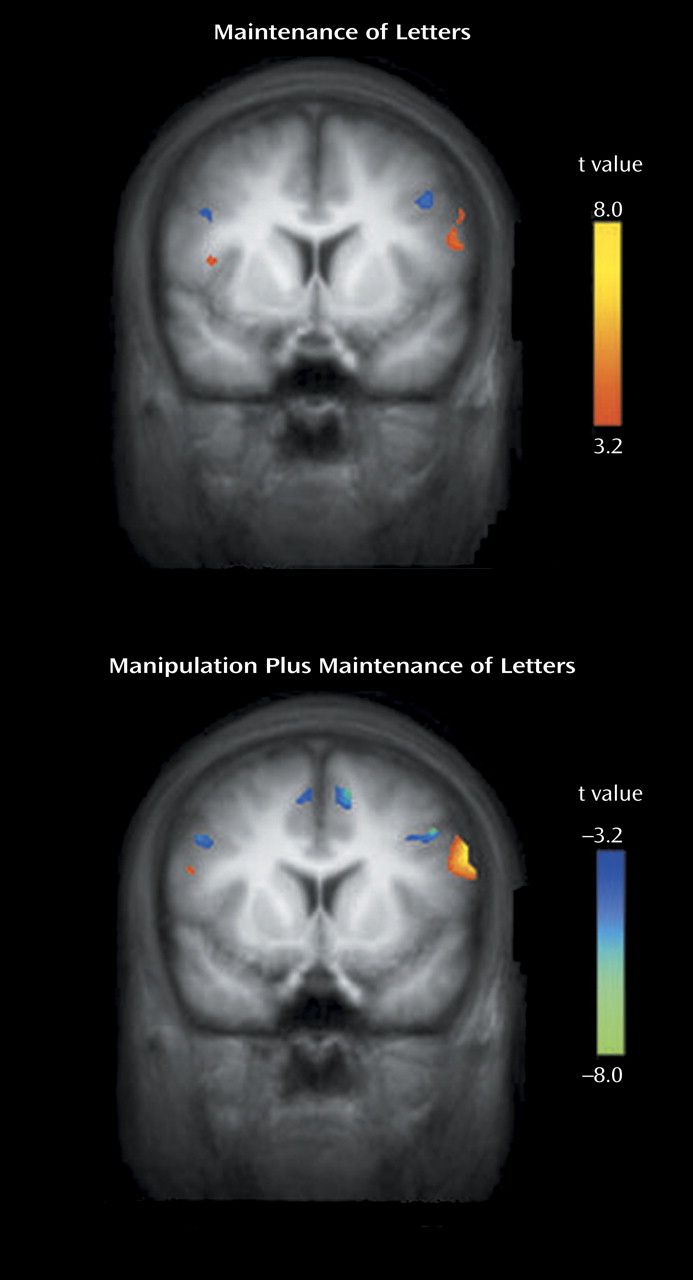
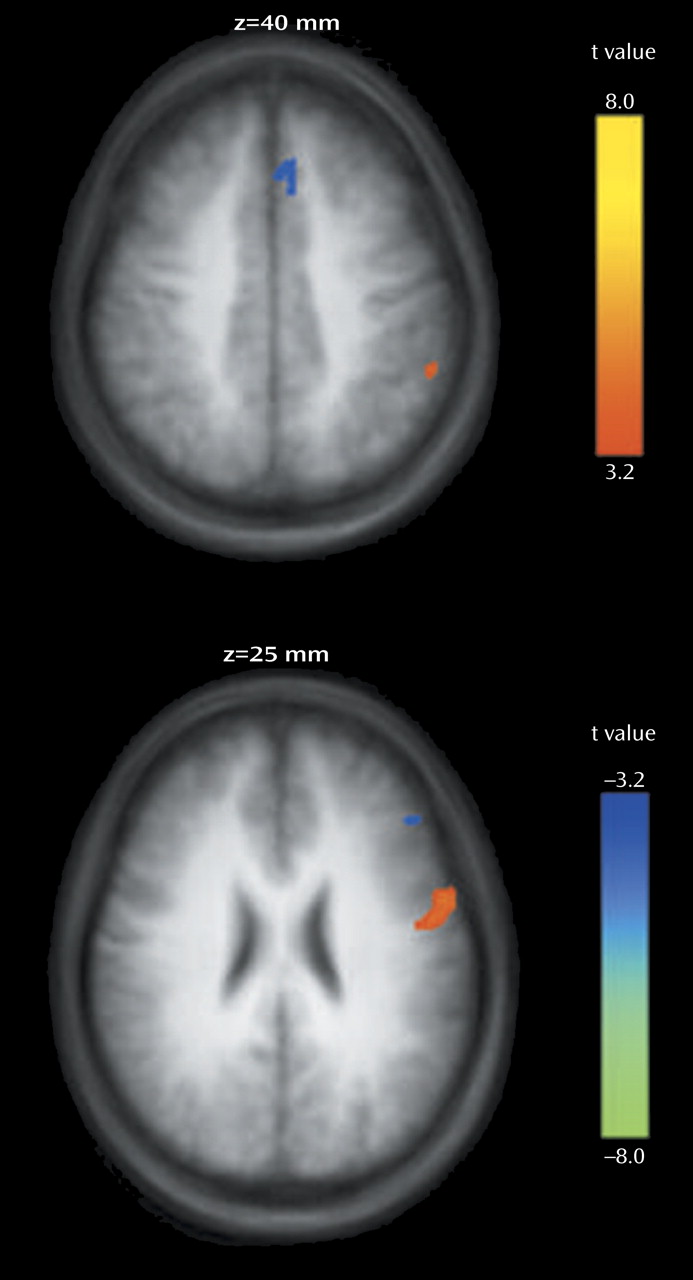
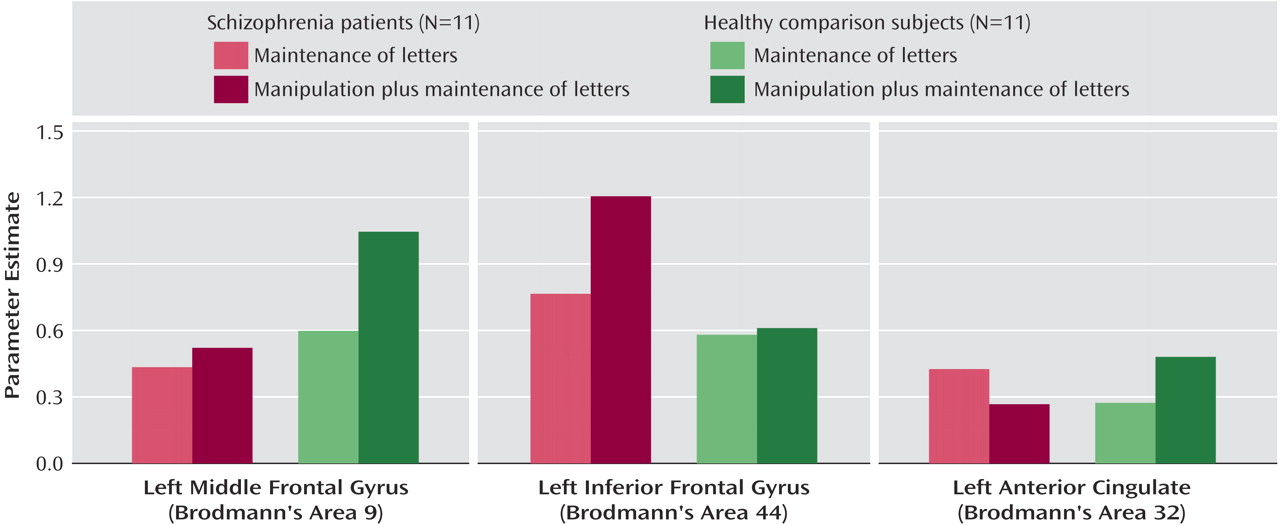
We found that both first-episode schizophrenia patients and healthy comparison subjects activated a predominantly left-sided frontal-parietal network, with the manipulation plus maintenance task eliciting activation of greater magnitude and spatial extent, compared to the maintenance task. In the patients, relative to the comparison subjects, both the maintenance and manipulation components of verbal working memory were associated with decreased activation in the dorsolateral prefrontal cortex and increased activation in the ventrolateral prefrontal cortex. In addition, these findings were more prominent in the manipulation component, as shown by the group-by-task interactions found for activation in the left dorsolateral prefrontal cortex and ventrolateral prefrontal cortex (
Figure 5).
Our finding of relative increases and decreases in prefrontal activation that were accentuated in manipulation versus maintenance tasks in patients adds to recent behavioral data suggesting that while both maintenance and central executive aspects of working memory are impaired in schizophrenia, the central executive aspect is more severely affected
(20). Several sources of behavioral findings have also suggested that schizophrenia patients are particularly impaired on tasks requiring executive processes
(28,
29). In concert with these studies, a recent study found working memory to be a core deficit that could be rate-limiting for a number of diverse cognitive functions affected in schizophrenia
(2). The present study adds functional neuroanatomical evidence to suggest that within working memory processes, manipulation may contribute more to dysfunction in schizophrenia. Supporting the present findings, Honey et al.
(30) reported that manipulation processes elicited greater BOLD signal change in a similar frontal-parietal network in healthy subjects exposed to subdissociative doses of ketamine; these findings were obtained in the context of a model of
N-methyl-
d-aspartic acid receptor hypofunction in schizophrenia.
The relatively lower dorsolateral prefrontal cortex activation during manipulation in patients adds support to several pieces of evidence for the importance of the dorsolateral prefrontal cortex in the pathophysiology of schizophrenia
(16,
31). Decreased dorsolateral prefrontal cortex activation and dysfunction have been demonstrated in several working memory fMRI studies of chronic schizophrenia
(32,
33), as well as first-episode schizophrenia
(4). In addition, a proton magnetic resonance spectroscopy study of schizophrenia patients showed that reduced dorsolateral prefrontal cortex
N-acetylaspartate concentrations correlate with abnormal activation within the working memory network
(6). Finally, postmortem studies have shown increased neuronal density in the dorsolateral prefrontal cortex in patients with schizophrenia
(34–
36).
In contrast to relatively decreased dorsolateral prefrontal cortex activation, we found a disproportionate increase in ventrolateral prefrontal cortex activation in first-episode patients during the manipulation task, compared to the maintenance task, suggesting that the ventrolateral prefrontal cortex might serve a compensatory function. Greater ventrolateral prefrontal cortex activation was also observed in another group of schizophrenia patients
(8). In healthy subjects, subvocal rehearsal and phonological loop processes are thought to contribute to activation of the left ventrolateral prefrontal cortex in verbal working memory tasks
(18,
37). In patients, the relatively greater activation observed might represent the overuse of this strategy in a neural substrate that is compensating for the dysfunctional dorsolateral prefrontal cortex. A synthesis with recent findings of cytoarchitectonic abnormalities selectively at the dorsolateral prefrontal cortex but not the ventrolateral prefrontal cortex
(38) further suggests that these activation differences may represent interacting regions of dysfunctional and compensatory neuroplastic activity in the dorsolateral prefrontal cortex and the ventrolateral prefrontal cortex, respectively.
Although we found relatively increased activation in the ventrolateral prefrontal cortex, others have found no difference
(4) or a decrease in activation
(9) in this region. These differences may be explained by variations in the study task or in patients’ medications or illness duration. For example, a prior study
(4) used a context processing task that differed from ours in that the subvocal rehearsal and the phonological loop were not taxed. The patients in the study that reported a decrease in activation
(9) were older and had a longer duration of illness, making the results of that study difficult to compare with ours.
Task-specific activity differences in patients versus comparison subjects were also observed in the left anterior cingulate. Whereas the healthy comparison subjects had greater activation in this region during the manipulation task, patients had less activation (
Figure 5). Given the close functional relationship between this region and the dorsolateral prefrontal cortex, and its complementary role in cognitive control and conflict monitoring, this reduced activation could contribute to the overall dysfunction in the prefrontal network in schizophrenia
(39).
An alternative explanation for our prefrontal activation findings is that they resulted from increased overall working memory load and/or difficulty involved in the manipulation task rather than from the manipulation per se. This explanation is less likely because the maintenance and manipulation tasks yielded similar behavioral results in both the patients and the comparison subjects in our study (see Results) and in an earlier study in which the participants were healthy subjects
(19).
The effects of atypical antipsychotic medications, which were taken by all of the patients in our study, deserve mention. We chose to study cognitive deficits after initial stabilization, because deficits that are present at that stage have been demonstrated to be less transient
(40), more reflective of the core pathology, and more predictive of functional outcome
(41). Although our finding of reduced dorsolateral prefrontal cortex activation is consistent with the findings of an earlier study that included neuroleptic-naive first-episode schizophrenia patients
(4), it is possible that medication effects contributed to the increased ventrolateral prefrontal cortex activation, as suggested by an fMRI study showing greater prefrontal cortex activation after substitution of risperidone for haloperidol in patients with schizophrenia
(42). This possibility, together with the broader question of prefrontal responses to medications in patients with early illness, is intriguing and warrants future investigation.
Finally, we note that these task-specific prefrontal findings were present in first-episode patients and therefore could have been established relatively early in the course of schizophrenia, at or before the first psychotic episode. These findings are unlikely to be a result of chronic illness and related epiphenomena. Although our findings support the notion that dorsolateral prefrontal cortex dysfunction is already present at the first psychotic episode, additional research will be needed to examine the nature of the ventrolateral prefrontal cortex response in relation to its possible compensatory role in information processing, the effect of parametric variation in task load on these brain activations, and the effect of medications and disease progression. We also need a better understanding of the relationship of these prefrontal findings to neuropsychological and epidemiological findings of cognitive deficits sustained before the onset of psychosis
(17,
43–45).