Methamphetamine, a psychostimulant with reinforcing effects similar to those of cocaine
(1), has reemerged as a major drug of abuse worldwide and is commonly used among HIV-infected individuals. In Thailand, both inhalation and injection of methamphetamine are associated with higher risk for HIV infection
(2). Similarly, studies and large surveys in the United States (California and New York) consistently found that even noninjection methamphetamine use contributes to higher rates of unprotected sexual activity in both men and women and to the risk of contracting sexually transmitted diseases, including HIV
(3,
4). Conversely, the rate of stimulant use is four times higher in men who have sex with men (who are at high risk for HIV), compared to heterosexual men, according to the U.S. National Household Survey on Drug Abuse
(5). Therefore, HIV and use of methamphetamine have become a “double epidemic” over the past decade
(4).
Like individuals with HIV-associated dementia, methamphetamine users have been reported to have slower reaction times, poorer decision-making abilities, and poorer performance on measures of memory, attention, and concentration, compared to non-drug users
(6,
7). A postmortem study
(8) and positron emission tomography (PET) studies
(9,
10) consistently found lower concentrations of dopamine transporter in methamphetamine users, compared to subjects with no history of methamphetamine use, but the differences in dopamine transporter levels were smaller (20%–30%) than those observed in animals treated with methamphetamine (>50%) or in patients with Parkinson’s disease (36%–71%)
(11). Although methamphetamine users typically do not have clinically evident extrapyramidal signs, one study found them to have slower performance on computerized tests that measure psychomotor speed and working memory, relative to age- and gender-matched comparison subjects
(12). The lower concentrations of dopamine transporter measured with PET were also associated with slower motor function (timed gait) and poorer recall (Auditory Verbal Learning Test scores)
(10). Because HIV viral burden is often highest in the basal ganglia, where the highest density of dopaminergic terminals is located, it is reasonable to hypothesize that the toxic effects of methamphetamine on the dopaminergic terminals may exacerbate HIV-associated brain injury in the basal ganglia.
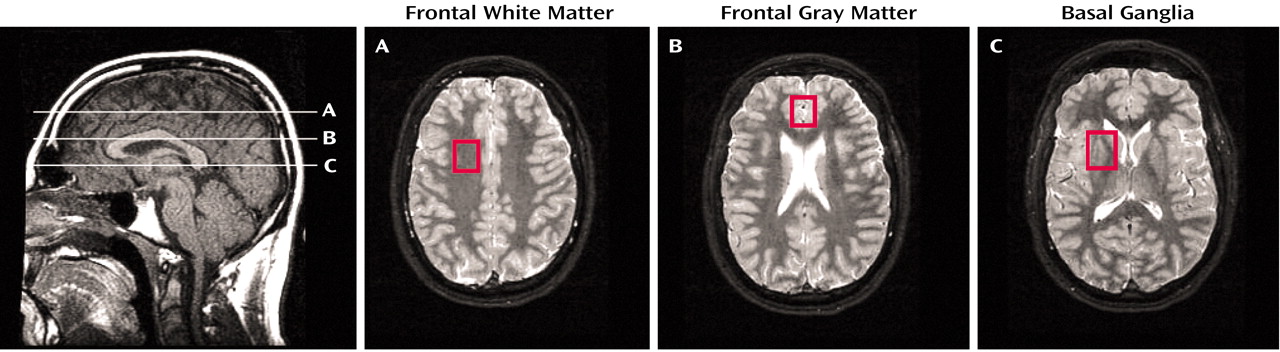
Proton magnetic resonance spectroscopy (
1H-MRS) documented lower concentrations of
N-acetyl compounds (
N-acetylaspartate) and total creatine in the basal ganglia
(13,
14) and higher concentrations of choline compounds and
myo-inositol in the frontal cortex in abstinent methamphetamine-dependent subjects
(13), compared with healthy non-drug users. These findings suggested long-term neuronal damage (with lower concentrations of
N-acetylaspartate) and glial abnormalities (with higher concentrations of
myo-inositol and choline compounds) in abstinent methamphetamine users. Numerous studies in patients with HIV dementia also found lower concentrations of the neuronal marker
N-acetylaspartate, especially in those with moderate to severe dementia, and higher concentrations of the glial marker
myo-inositol or choline compounds that are related to dementia severity
(15,
16). Therefore,
1H-MRS may provide useful surrogate markers to assess the combined effects of methamphetamine and HIV on the brain.
To determine whether HIV and methamphetamine have an additive effect on brain metabolite abnormalities, we performed 1H-MRS in four groups of subjects: HIV-positive subjects with no history of drug dependence, HIV-positive subjects with a history of methamphetamine dependence or abuse, HIV-negative subjects with a history of methamphetamine dependence or abuse, and HIV-negative subjects with no history of drug dependence. We hypothesized that 1) neurochemical abnormalities, particularly lower concentrations of N-acetyl compounds (N-acetylaspartate), would be more evident in HIV-positive subjects with a history of chronic methamphetamine use, compared to subjects with either HIV or chronic methamphetamine use alone, and 2) that the basal ganglia brain region would be affected most severely (lowest N-acetylaspartate concentrations), because this region contains the highest concentrations of dopaminergic nerve terminals.
Results
Clinical and Demographic Variables
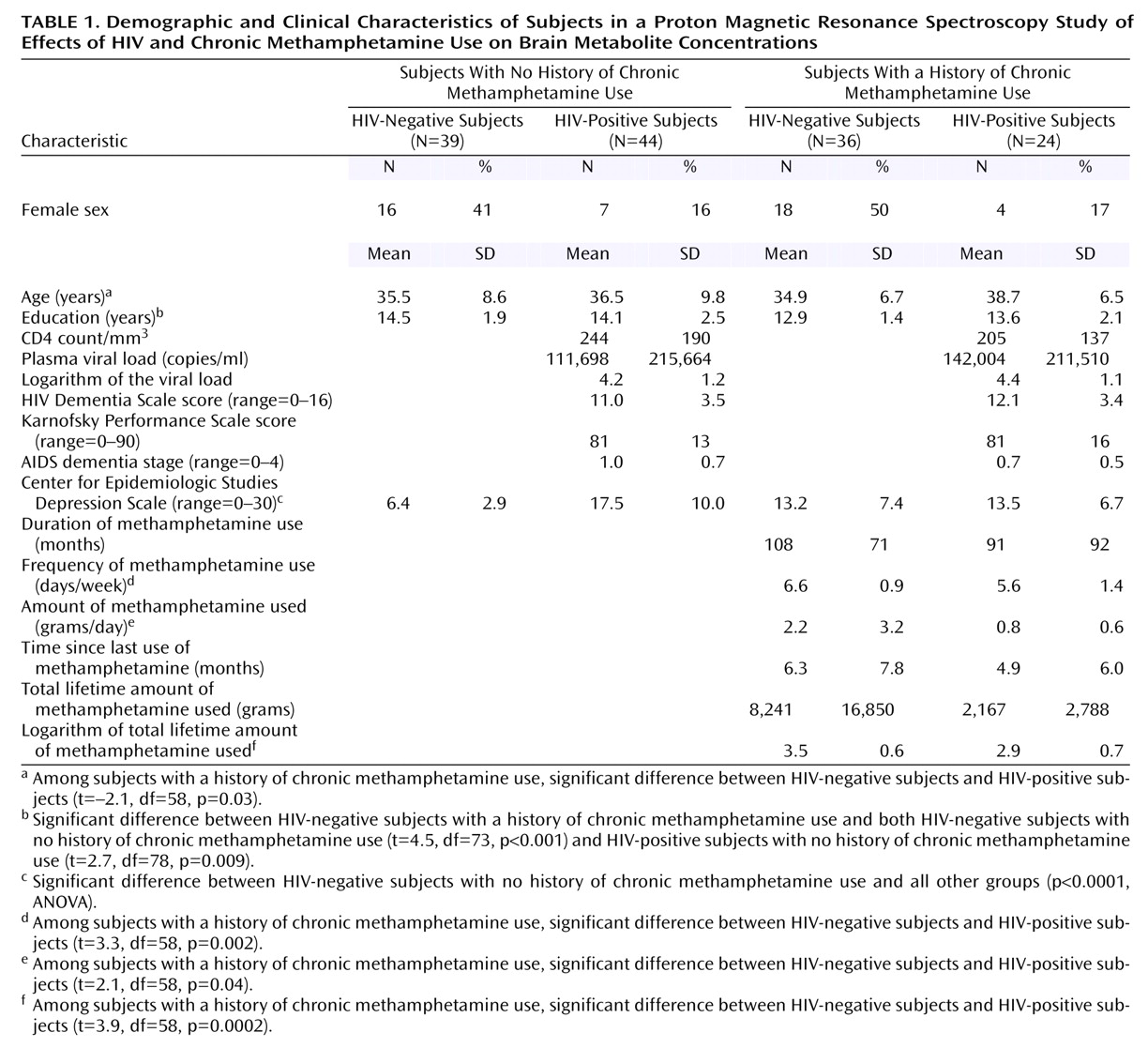
Table 1 summarizes the demographic characteristics and clinical variables related to HIV disease severity or methamphetamine use in the respective subject groups. No significant difference in age was found between the groups. The two HIV-positive groups included fewer female subjects, compared to the two HIV-negative groups. However, the two HIV-positive groups had similar HIV disease severity, as measured by CD4 count, plasma viral loads, Karnofsky Performance Scale scores, and AIDS dementia stage. The HIV-negative subjects with no history of chronic methamphetamine use had significantly lower scores for depressive symptoms, as measured by the Center for Epidemiologic Studies Depression Scale, compared to the other three groups (p<0.0001, Fisher’s PSLD test). A significant difference in educational level was found between groups (p=0.002, ANOVA); the difference was due to the lower level of education in the HIV-negative subjects with a history of chronic methamphetamine use, compared to the two groups with no history of chronic methamphetamine use, but the educational levels of two groups with a methamphetamine use history were not significantly different.
Methamphetamine and Other Drug Use
The two groups with a history of chronic methamphetamine use had similar durations of methamphetamine use. However, because the HIV-positive subjects with a methamphetamine use history used the drug in smaller amounts (t=2.1, df=58, p=0.04) and at a lower frequency (t=3.3, df=58, p=0.002) than the HIV-negative subjects, their total lifetime methamphetamine intake was also lower (log transformed t=3.9, df=58, p=0.0002). Significantly greater nicotine use (p≤0.001, Fisher’s PLSD test) was observed in both groups with a history of chronic methamphetamine use (HIV-negative subjects: mean=14.6 pack-years, SD=15; HIV-positive subjects: mean=13.5 pack-years, SD=12), compared to the non-drug-user groups (HIV-negative subjects: mean=3.8 pack-years, SD=10; HIV-positive subjects: mean=4.7 pack-years, SD=9). Although subjects with a history of other drug dependence, including alcohol dependence, were excluded, 20 (83%) HIV-positive subjects with a history of chronic methamphetamine use, 16 (44%) HIV-negative subjects with a history of chronic methamphetamine use, and four (9%) HIV-positive subjects with no history of chronic methamphetamine use also admitted to past recreational or occasional use of cocaine. In addition, 20 (55%) HIV-negative subjects with a history of chronic methamphetamine use, 13 (54%) HIV-positive subjects with a history of chronic methamphetamine use, 18 (41%) HIV-positive subjects with no history of methamphetamine use, and 11 (28%) HIV-negative subjects with no history of methamphetamine use also used marijuana occasionally. The amount of alcohol used was less than 7 drinks/week in all subjects except two HIV-negative subjects with a history of chronic methamphetamine use. All subjects with a history of chronic methamphetamine use indicated that methamphetamine was their primary drug of abuse and their drug of choice and that their use of other drugs was minimal, compared to the use of methamphetamine.
Group Differences in Brain Metabolites
HIV infection (independent of methamphetamine use), compared with seronegative status, was associated with mildly lower concentrations of N-acetylaspartate in the frontal gray matter (–5.3% difference in concentration; F=11.1, df=1, 121, p=0.001) and basal ganglia (–5.0%; F=8.6, df=1, 128, p=0.004) but not in the frontal white matter (–2.6%; F=2.2, df=1, 128, p=0.10), mildly lower concentrations of creatine in the basal ganglia (–3.7%; F=4.6, df=1, 127, p=0.03) and the frontal gray matter (–3.9%; F=4.7, df=1, 121, p=0.03), and higher concentrations of choline compounds (7.1%; F=9.3, df=1, 129, p=0.003) and myo-inositol (7.1%; F=6.1, df=1, 129, p=0.01) in the frontal white matter. In contrast, a history of chronic methamphetamine use (independent of HIV serostatus), compared with no history of chronic methamphetamine use, was associated with a mildly lower concentration of N-acetylaspartate in the basal ganglia (–3.5% difference in concentration; F=4.6, df=1, 128, p=0.03), lower concentrations (approaching significance) of creatine in the basal ganglia (–3.1%; F=3.3, df=1, 127, p=0.07) and of N-acetylaspartate in the frontal white matter (–3.8%; F=2.7, df=1, 128, p=0.1), higher concentrations of choline compounds in both the frontal white matter (4.5%; F=4.9, df=1, 129, p=0.03) and the frontal gray matter (9.8%; F=7.2, df=1, 121, p=0.008), and a higher concentration of myo-inositol in the frontal gray matter (9.0%; F=7.0, df=1, 120, p=0.009). ANOVA showed no significant interactions on any of the 1H-MRS variables.
Because the concentrations of several metabolites showed education effects (basal ganglia choline compounds, frontal white matter choline compounds, and basal ganglia myo-inositol) and age effects (frontal white matter choline compounds and basal ganglia myo-inositol), we adjusted for these variables in further analyses. After adjustments for age and education with ANCOVA, the higher concentration of frontal white matter choline compounds initially observed in the HIV-positive subjects and the subjects with a history of chronic methamphetamine use was no longer significant; however, the other results were unaffected. The greater proportion of men in the HIV-positive groups might have further contributed to the higher concentration of frontal white matter choline compounds in those groups, as the concentration of choline compounds was 7% higher in men than in women among the HIV-negative subjects with no history of chronic methamphetamine use (p=0.08, Fisher’s PLSD test).
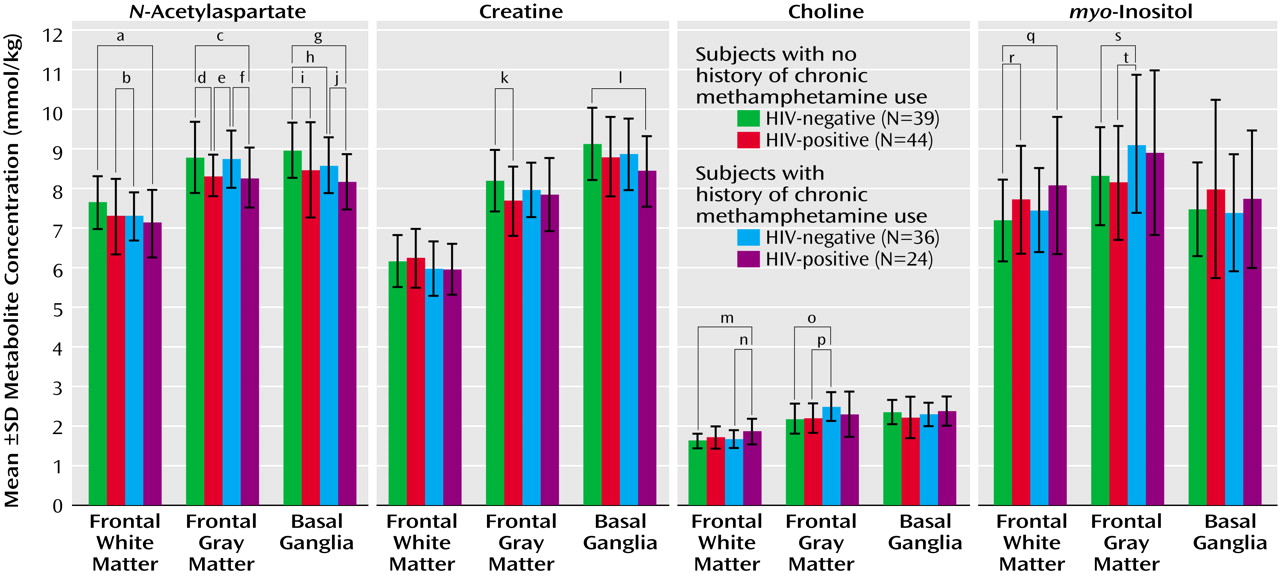
The combined effects of HIV infection and chronic methamphetamine use were generally consistent with an additive model. As a result, metabolite abnormalities commonly were greatest in HIV-positive subjects with a history of chronic methamphetamine use (
Figure 2). The synergistic effect was particularly pronounced for the neuronal marker
N-acetylaspartate, in that HIV-positive drug users consistently had the lowest concentration of
N-acetylaspartate of all four subject groups. Post hoc test findings for the HIV-positive methamphetamine users, compared to the HIV-negative subjects with no history of chronic methamphetamine use, showed lower concentrations of
N-acetylaspartate in the frontal white matter (–6.1% difference in concentration; t=2.3, df=57, p=0.02), the frontal gray matter (–5.7%; t=2.2, df=60, p=0.03), and the basal ganglia (–9%; t=4.2, df=60, p=0.0001). In addition, the HIV-positive subjects with a history of chronic methamphetamine use had the lowest concentration of creatine in the basal ganglia (–7.4%; t=2.8, df=60, p=0.007) and the highest concentrations of choline compounds (14.5%; t=3.5, df=57, p=0.0009) and
myo-inositol (12.2%; t=2.4, df=56, p=0.02) in the frontal white matter. However, the higher concentration of frontal white matter choline compounds was no longer significant after adjustment for age and sex differences between the groups.
Correlations Between Metabolite Concentrations, Drug Use, and HIV Disease Severity
Metabolite concentrations did not correlate with methamphetamine use variables (frequency of use, amount used, duration of use, or cumulative dose) or with other drug use variables (pack-years of cigarettes, cumulative lifetime amount of alcoholic drinks, lifetime amount of cocaine, or lifetime joints of marijuana). Most of the HIV disease severity measures correlated with 1H-MRS metabolite concentrations. CD4 count correlated with concentrations of choline compounds in the frontal gray matter (r=–0.38, p=0.006, N=56), choline compounds in the frontal white matter (r=–0.26, p=0.05, N=56), myo-inositol in the frontal gray matter (r=–0.41, p=0.003, N=51) and myo-inositol in the frontal white matter (r=0.55, p<0.0001, N=56). The correlation of the logarithm of the viral load with the concentration of N-acetylaspartate in the basal ganglia approached significance (r=–0.25, p=0.08, N=50), and Karnofsky Performance Scale scores correlated inversely with concentrations of choline compounds in all three brain regions (frontal cortex: r=–0.48, p=0.0003, N=52; frontal white matter: r=–0.34, p=0.01, N=55; basal ganglia: r=–0.33, p=0.01, N=57), as well as inversely with concentrations of myo-inositol in all three brain regions (frontal cortex: r=–0.56, p<0.0001, N=51; frontal white matter: r=–0.35, p=0.009, N=55; basal ganglia: r=–0.3, p=0.03, N=56). In addition, AIDS dementia stage correlated with concentrations of creatine in the frontal white matter (r=0.27, p=0.04, N=60), myo-inositol in the basal ganglia (r=0.33, p=0.01, N=60), and myo-inositol in the frontal gray matter (r=0.36, p=0.009, N=51). These clinical variables, however, did not correlate with any of the self-reported drug use variables.
Discussion
This study shows that the injurious effects of HIV and chronic methamphetamine use on the brain are generally additive, with respect to both neuronal and glial
1H-MRS markers. First, the HIV-positive subjects with a history of chronic methamphetamine use had the lowest concentration of
N-acetylaspartate in all three brain regions studied, compared to the other three subject groups; this finding suggests significant neuronal loss or dysfunction in these brain regions. As predicted, the region most affected was the basal ganglia, which has the highest density of dopaminergic nerve terminals. Although a lower concentration of
N-acetylaspartate was detected in a group of subjects with methamphetamine use alone
(13), prior
1H-MRS studies typically detected minimal or no differences in
N-acetylaspartate concentration in HIV patients who had mild dementia or were neuroasymptomatic
(16,
23). In the current study, the HIV-positive subjects with and those without a history of chronic methamphetamine use both had mild dementia; therefore, any differences in
N-acetylaspartate concentrations in those groups, compared with the HIV-negative subjects, should have been minimal. However, the
N-acetylaspartate concentration in the HIV-positive subjects with a history of chronic methamphetamine use was almost twofold smaller than that in subjects with HIV or chronic methamphetamine use alone, despite similar dementia severity in the HIV-positive subjects with and without a history of chronic methamphetamine use and the lower level of methamphetamine use in the HIV-positive subjects, compared with the HIV-negative subjects. Possibly because of the variability in the dementia status of the HIV-positive subjects, however, the difference between the HIV-positive subjects with no history of chronic methamphetamine use and those with a history of chronic methamphetamine use did not reach statistical significance. We also found a lower concentration of creatine in the basal ganglia in the HIV-positive subjects with a history of chronic methamphetamine use, which parallels the finding of a lower concentration of
N-acetylaspartate and is also consistent with possible neuronal injury, because creatine is present in both neurons and glia. The findings for
N-acetylaspartate in the HIV-positive subjects with a history of chronic methamphetamine use are consistent with reports from a prior, but preliminary
1H-MRS study in which lower concentrations of
N-acetylaspartate only and no differences in other metabolites were found in the frontal lobes of HIV-infected and stimulant-dependent individuals, compared to subjects with either condition alone
(24). In that report, however, the stimulant drug(s) and the amounts used were not specified and the number of subjects was substantially smaller (three to seven subjects per group) than in the current study.
Neuropathological evidence for neuronal loss or injury is available only from studies performed in subjects with either methamphetamine use only or HIV only. A postmortem study of relatively young methamphetamine users found lower concentrations of dopamine nerve terminal markers (dopamine, tyrosine hydroxylase, and the dopamine transporter) and normal levels of dopa decarboxylase and vesicular monoamine transporter in the striatum
(8). In HIV-infected brains, neuronal apoptosis and consequent neuronal loss are well described
(25). Therefore, combined neuronal injury related to methamphetamine use and HIV, as observed in additive effects on
N-acetylaspartate concentration, would be expected.
Although the exact mechanisms of brain injury underlying the interaction between HIV and methamphetamine are unclear, HIV-1 Tat protein and methamphetamine synergistically interacted to reduce striatal dopaminergic function (up to a fivefold reduction) in an in vivo rodent model
(26). One possible pathway to the combined neurotoxicity might be a methamphetamine-induced increase in extracellular dopamine, which in turn would activate HIV replication. Dopamine can increase viral replication in HIV-infected T-lymphoblasts
(27), and dopaminergic agents (selegiline and
l-dopa) might enhance CNS pathology and viral replication, as shown in a simian immunodeficiency virus model
(28). Methamphetamine itself also increased replication of feline immunodeficiency virus in cultured feline astrocytes
(29). Likewise, cocaine, which was abused occasionally by the majority of the HIV-positive subjects with a history of chronic methamphetamine use in the current study, might further enhance HIV replication
(30), although cocaine’s direct effect on
N-acetylaspartate would be minimal
(31). Thus, the intermittently increased viral burden in the combined condition might exacerbate brain injury.
Data regarding the combined effects of HIV and psychostimulant abuse on the dopaminergic system in humans are extremely limited. Because many antiretrovirals are potent inhibitors of cytochrome P450 systems, HIV patients taking protease inhibitors have experienced prolonged effects of psychostimulants, fatal interactions with 3,4-methylenedioxymethamphetamine
(32), and possible fatal interactions with methamphetamine
(33). In a case report, an HIV-positive psychostimulant (methamphetamine and cocaine) user showed rapid decline of cognitive function and an unusual movement disorder; only the motor disorder responded to dopamine replacement
(34). No human studies have specifically evaluated the interactive effects of HIV and chronic methamphetamine use on the dopaminergic system.
In this study, the HIV-negative subjects with a history of chronic methamphetamine use (who had an average of 6 months of abstinence) had higher concentrations of choline compounds and
myo-inositol in the frontal cortex (gray matter). These findings suggest an inflammatory response, or glial activation, because these metabolites are found in much higher concentrations in glial cells than in neurons
(35), and are consistent with findings of acute methamphetamine-induced glial activation in rodents
(36) and monkeys
(37), as well as findings of chronic effects in monkeys up to 1.5 years after methamphetamine exposure
(37). Methamphetamine, like HIV, has been reported to stimulate the production of the inflammatory cytokine, tumor necrosis factor α
(38), and to increase binding and activation of the redox-responsive transcription factors, AP-1 and NF-kappaB
(39). These factors may be associated with the inflammatory findings (higher concentrations of choline compounds and
myo-inositol) observed with
1H-MRS.
In the HIV-positive subjects with a history of chronic methamphetamine use in the current study, the frontal white matter showed additive effects of higher concentrations of the glial metabolite
myo-inositol, compared to the subjects with either HIV or chronic methamphetamine use alone. This finding suggests a synergistic effect of HIV and methamphetamine on reactive glial processes in the frontal white matter, as higher concentrations of glial metabolites were observed both in the current study and in prior
1H-MRS studies of HIV patients
(16) and of abstinent methamphetamine-dependent subjects
(13). Microglial activation
(40) and neuronal apoptosis
(41) in HIV encephalitis, as well as the effects of the synergistic interaction between drug use (primarily opiates) and advanced HIV infection on inflammatory infiltrates
(40) and microglial activation
(42), have been well documented. However, little is known about how the HIV-infected brain responds to chronic methamphetamine use. Because excessive levels of dopamine related to chronic methamphetamine use may stimulate HIV replication
(27), increased concentrations of inflammatory glial markers may be a response to the intermittently higher levels of viral burden in the HIV-positive subjects with a history of chronic methamphetamine use, as well as a direct effect of methamphetamine use. In the basal ganglia, however, no differences between study groups in concentrations of choline compounds or
myo-inositol were observed. These findings suggest the lack of inflammatory or repair responses in the basal ganglia, which might lead to the lower concentration of
N-acetylaspartate.
Preclinical studies indicate that methamphetamine affects the dopaminergic system, causing massive release of dopamine acutely, whereas chronic methamphetamine abusers showed down-regulation of dopamine transporters and postsynaptic D
2 receptors, as observed during early abstinence
(10,
43). However, significant recovery of both the dopamine transporter and D
2 receptors occurs in protracted abstinence in nonhuman primates
(37) and in humans
(43). This recovery may be mediated by activated macrophages and more so by microglia, which express brain-derived neurotrophic factor mRNA to stimulate dopaminergic sprouting, as shown in response to striatal injury in a lesioned mouse model
(44). Both macrophages and microglia quickly accumulated near the wound site after striatal injury and may persist for the long term
(44). Therefore, subjects with a history of chronic methamphetamine use may demonstrate an increased glial response even during protracted abstinence. The current study found higher concentrations of glial metabolites primarily in the frontal cortex of HIV-negative subjects with a history of chronic methamphetamine use and in the frontal white matter of the HIV-positive subjects with a history of chronic methamphetamine use.
These findings are similar to those of some preclinical MRS studies. An ex vivo
1H-MRS study that evaluated the effects of feline immunodeficiency virus and methamphetamine in the brain found that higher concentrations of choline compounds in the frontal white matter and higher levels of γ-aminobutyric acid were associated with methamphetamine, but lower concentrations of choline compounds were associated with feline immunodeficiency virus, and no additive effects resulting in lower concentrations of
N-acetylaspartate were associated with both methamphetamine and feline immunodeficiency virus
(45). Lower levels of glutamate were found in one ex vivo MRS study of feline immunodeficiency virus
(45), but higher levels were found in another
(46). However, the two studies differed in viral strain, route of infection, time of infection, and possibly differences in the brain region(s) that were measured. Such animal models can measure the exact doses of drugs used and the duration of HIV infection, which are difficult to assess precisely in humans. This lack of precision might be one reason we did not find correlations between
1H-MRS metabolite levels and self-reported drug use. However, most preclinical studies cannot model the longer duration of HIV infection or methamphetamine exposure in humans. Furthermore, because the metabolism of methamphetamine is significantly different among species and because there are differences in pathogenesis between the lentiviruses in the preclinical models (feline and simian immunodeficiency virus) and HIV, studies such as the current study are needed to assess the combined effects of methamphetamine and HIV in humans.
Postmortem human studies are also valuable, but the ability to study a large group of subjects in postmortem studies is extremely limited, and much of the in vivo pathophysiology may not be evident or may be altered postmortem. This study demonstrates that 1H-MRS is a robust and sensitive method for assessing in vivo pathophysiology. Future studies using other noninvasive neuroimaging techniques, such as dopamine markers on PET and functional MRI, may provide further insights into the in vivo pathophysiological changes associated with the combined effects of HIV and methamphetamine and may help to determine whether treatment with medications or cognitive behavior therapy alters brain function and pathology. The current findings of metabolite abnormalities support an additive effect of HIV and methamphetamine on brain injury, especially in the striatal and frontal brain regions that have the highest density of dopaminergic nerve terminals.