The lifetime prevalence of bipolar disorder is between 1% and 3% and may be present in children and adolescents
(1,
2). Attention deficit hyperactivity disorder (ADHD) has been reported in 70% to 98% of children with bipolar disorder
(2). The ability to distinguish bipolar disorder, ADHD, and ADHD with comorbid bipolar disorder is important in terms of treatment management because bipolar disorder and ADHD require different treatment strategies
(3,
4).
Proton magnetic resonance spectroscopy (
1H MRS) can measure a number of cerebral metabolites, including creatine plus phosphocreatine (Cr),
myo-inositol-containing compounds (Ino:
myo-inositol with small contributions from inositol-1-phosphate
[5]), and glutamate plus glutamine (Glx).
1H MRS has shown a higher Ino-to-Cr ratio in the anterior cingulate cortex and frontal cortex gray matter of children with bipolar disorder than in healthy comparison subjects
(6–
8). In contrast,
1H MRS has shown a higher Glx-to-Cr ratio in the prefrontal cortex and frontal lobe of children with ADHD than in healthy comparison subjects
(9,
10).
We used
1H MRS to investigate anterior cingulate cortex metabolite levels in children and adolescents with ADHD alone, ADHD plus bipolar disorder, and no psychiatric diagnosis. Some of the healthy comparison subjects were siblings of the children with ADHD. Siblings were chosen in an effort to distinguish state differences between children with ADHD, those with ADHD plus bipolar disorder, and healthy comparison subjects. Using
1H MRS, Chang et al.
(11) measured trait differences in the prefrontal cortex of children genetically at risk for bipolar disorder. On the basis of the literature, we hypothesized that a higher Glx-to-Cr ratio would be associated with ADHD and a higher Ino-to-Cr ratio would be associated with ADHD plus bipolar disorder; thus the Glx-to-Ino ratio could distinguish ADHD from ADHD plus bipolar disorder.
Method
This study was approved by the Institutional Review Boards of Massachusetts General Hospital and McLean Hospital. All parents signed written informed consent forms and all children signed written informed assent forms. Subjects were recruited through the Pediatric Psychopharmacology Research Program at Massachusetts General Hospital. Fifteen children with ADHD, eight children with ADHD plus bipolar disorder, and seven healthy comparison children (four of whom were siblings of the children with ADHD) were recruited.
To be included in the study, subjects could be male or female between 6 and 17 years old. They met DSM-IV diagnostic criteria for either ADHD (inattentive, hyperactive/impulsive, or combined type) or ADHD plus comorbid bipolar disorder. Healthy comparison subjects did not meet criteria for any axis I diagnoses. A complete psychiatric history was obtained from all subjects by using a structured diagnostic interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children. None of the children studied had a learning disability. Seven of the children with ADHD and all of those with ADHD plus bipolar disorder met criteria for oppositional defiant disorder, two children with ADHD plus bipolar disorder met criteria for generalized anxiety disorder, and one child with ADHD plus bipolar disorder met criteria for conduct disorder.
Three children with ADHD and two with ADHD plus bipolar disorder were medicated at the time of the study. One child received amphetamine (4 months), bupropion (4 months), and sertraline (18 months); one child received atomoxetine (<1 month); one child received amphetamine (14 months); one child received clonazepam (10 months); and one child received clonazepam (4 years), desmopressin (2 years), and sertraline (7 months).
Inclusion and exclusion criteria were applied at the beginning of the study on the basis of psychiatric evaluation and questionnaires, a detailed medical history, information on consumptive habits, a drug screen, laboratory analyses, and a description of presenting and preexisting conditions.
Imaging and spectroscopy were performed with a 1.5-T General Electric Signa scanner (GE Medical Systems, Milwaukee). Anatomical images were acquired for segmentation and spectral localization
(5). Spectra were acquired from the anterior cingulate cortex by using Probe-P (GE Medical Systems) (echo time=30 msec, repetition time=2 seconds, 128 averages, nominal voxel size=2 cm×2 cm×1.2 [S-I cm]) and analyzed by using LCModel
(12). Metabolite ratios rather than absolute values were calculated and used because at this time we are unable to get reliable absolute values. The method for estimating voxel gray matter, white matter, and CSF is described elsewhere
(5).
Analyses of variance were used to test the hypotheses. A result of p<0.05 was considered significant.
Results
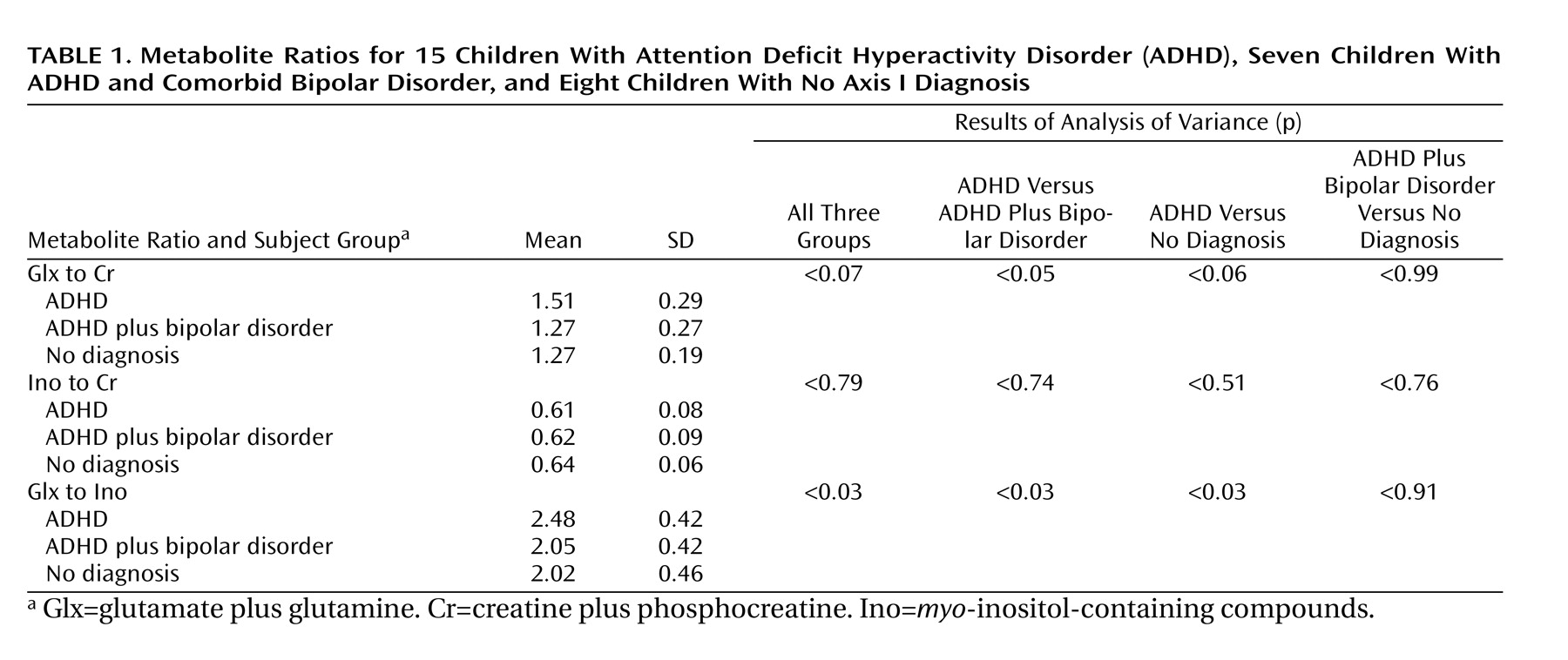
The Glx-to-Ino ratio differed among the three diagnostic groups (F=3.99, df=2, 27, p<0.03) (
Table 1). Subjects with ADHD had a significantly higher Glx-to-Ino ratio than subjects with ADHD plus bipolar disorder (mean difference=0.18, p<0.03) and healthy subjects (mean difference=0.19, p<0.03).
The Glx-to-Cr ratio differed among the three groups (F=2.95, df=2, 27, p<0.07); this difference was significant between children with ADHD and those with ADHD plus bipolar disorder (mean difference=0.24, p<0.05). The children with ADHD had a higher Glx-to-Cr ratio than healthy children (mean difference=0.24, p<0.06). There were no significant differences in Ino-to-Cr ratio among the three groups (F=0.23, df=2, 27, p<0.79).
There were no significant differences in voxel tissue composition among the three groups (gray matter: F=0.21, df=2, 22, p=0.81; CSF: F=3.31, df=2, 22, p=0.06; white matter: F=0.33, df=2, 22, p=0.72).
No significant differences were found in Glx-to-Cr ratio (F=0.06, df=1, 6, p=0.82), Ino-to-Cr ratio (F=0.00, df=1, 6, p=0.99), or Glx-to-Ino ratio (F=0.00, df=1, 6, p=1.00) between sibling and nonsibling healthy comparison subjects.
Discussion
Abnormal glutamate function in terminal areas of dopaminergic neurons have been hypothesized to contribute to the development of ADHD
(13). Methylphenidate and amphetamine affect dopamine and norepinephrine pathways crucial to frontal lobe function
(14) and have been shown to increase extracellular dopamine in the brain by blocking dopamine reuptake following glutamate-stimulated release of dopamine in the nucleus accumbens
(13,
15,
16). Dopamine acting on D4 receptors inhibits glutamate release from prefrontal cortical afferents in the nucleus accumbens
(13). One could speculate that reduced dopamine in the nucleus accumbens could lead to increased glutamate in the prefrontal cortex and that treatment with stimulants could, in turn, lead to reductions in glutamate in the prefrontal cortex.
Ino and inositol-1-phosphate are involved in the second messenger phosphatidylinositol cycle, which has been implicated in the pathophysiology of affective disorders
(5). Phosphatidylinositol circuits have also been implicated in the mechanism of action of lithium, valproate, and carbamazepine
(17,
18). The children with ADHD plus bipolar disorder had slightly lower Ino-to-Cr ratios than healthy subjects. This finding is discrepant with the finding of others
(6–
8) that subjects with bipolar disorder have higher Ino-to-Cr ratios than healthy subjects. All of the subjects in the study by others had bipolar disorder with comorbid ADHD, which could have affected the Ino-to-Cr ratios. Children with ADHD plus bipolar disorder are more resistant to lithium
(19) and valproate
(20) treatment than children with bipolar disorder alone. This could be attributable in part to differences in Ino metabolism between these two groups. Valproate, like lithium and carbamazepine, reduces brain inositol levels
(18).
Our subjects with ADHD had lower Ino-to-Cr ratios than the healthy comparison subjects. These results are consistent with clinical observations. Lithium and carbamazepine are inadvisable for children with ADHD
(21). One of the mechanisms of action of these drugs is to reduce brain inositol levels
(18). Such a reduction could further reduce the already low Ino levels in ADHD, leading to worsening of symptoms.
In conclusion, this study adds confirmation that ADHD may be a result of glutamatergic dysfunction in the anterior cingulate cortex. The 1H MRS Glx-to-Ino ratio could be used to differentiate between children and adolescents with ADHD who do or do not have comorbid bipolar disorder.