Social and occupational impairment has long been recognized as a core feature of schizophrenia affecting social interaction, vocational and instrumental functioning skills, self-care, and recreation
(1) . Poor performance on neurocognitive tasks has been widely observed to be associated with poor performance on measures of community functioning, psychosocial skill acquisition, and social problem solving
(2), and several domains of neuropsychological performance have been consistently associated with overall clinical outcomes, albeit less consistently in first-episode patients
(3 –
5) .
Both negative and positive symptoms of schizophrenia have also been found to be associated with functional outcomes. Few studies, however, have sought to compare the magnitude of the associations of neurocognition and symptoms with functioning, and the results that have been published have been somewhat conflicting
(6 –
10) . Some cross-sectional studies of chronic schizophrenia have suggested that psychopathology might be more strongly correlated with community functioning than cognition
(8,
11), while others have concluded that cognition is the strongest correlate
(2) .
A recent cross-sectional study
(12) using data from a large randomized Department of Veterans Affairs (VA) trial of antipsychotic medication for schizophrenia examined the association of measures of positive and negative symptoms, as well as of neurocognition, with community functioning as measured by both the Heinrichs-Carpenter Quality of Life Scale
(13) and days of employment. In that study of nonelderly, predominantly community-dwelling adults, the Positive and Negative Syndrome Scale (PANSS)
(14) measure of negative symptoms was modified to exclude items that directly related to cognitive capacity or functioning. Results suggested that 1) symptoms and neurocognition both have independent associations with functioning and 2) symptoms may be even more strongly associated with functioning than neurocognition. Further examination of the relationship of neurocognition and symptoms to functioning is needed and could potentially guide the development of pharmacological and rehabilitative treatments and further our understanding of the complexity of this illness.
In this study, we examined the relative strength of association of measures of community functioning, as assessed by the Quality of Life Scale and self-reported days of employment, with measures of both neurocognition and symptoms. The data were from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study, a large randomized, controlled trial funded by the National Institute of Mental Health and designed to compare outcomes of antipsychotic medications
(15) . Although CATIE aimed to compare the effectiveness of different medications over an 18-month follow-up period, investigators found few differences between treatments in their effect on symptoms
(16), neurocognition
(17), or community functioning but did note significant relationships of both baseline positive symptoms and neurocognition with improvement in community functioning
(18) .
Results
Subject Characteristics
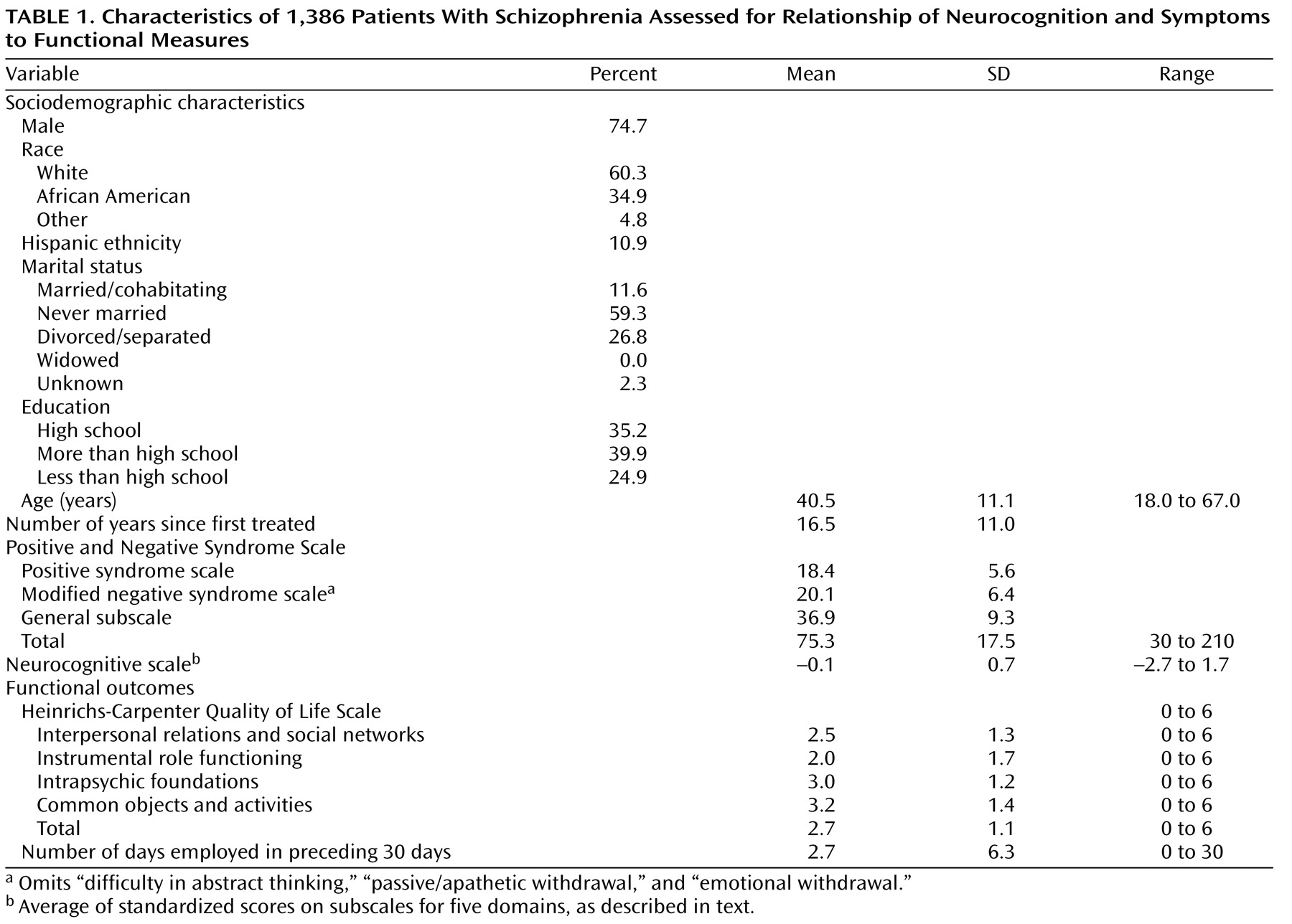
This analysis included 1,386 patients of the 1,432 individuals with a DSM-IV diagnosis of schizophrenia, validated by the SCID, who were treated at 57 U.S. sites. A total of 792 patients completed the 6-month follow-up, and 77 completed the 18-month follow-up. The patients were middle-aged, and most were Caucasian and chronically ill (
Table 1 ). The group was similar in sociodemographic and clinical characteristics to participants in other major trials of atypical antipsychotics
(30) .
Bivariate Analyses
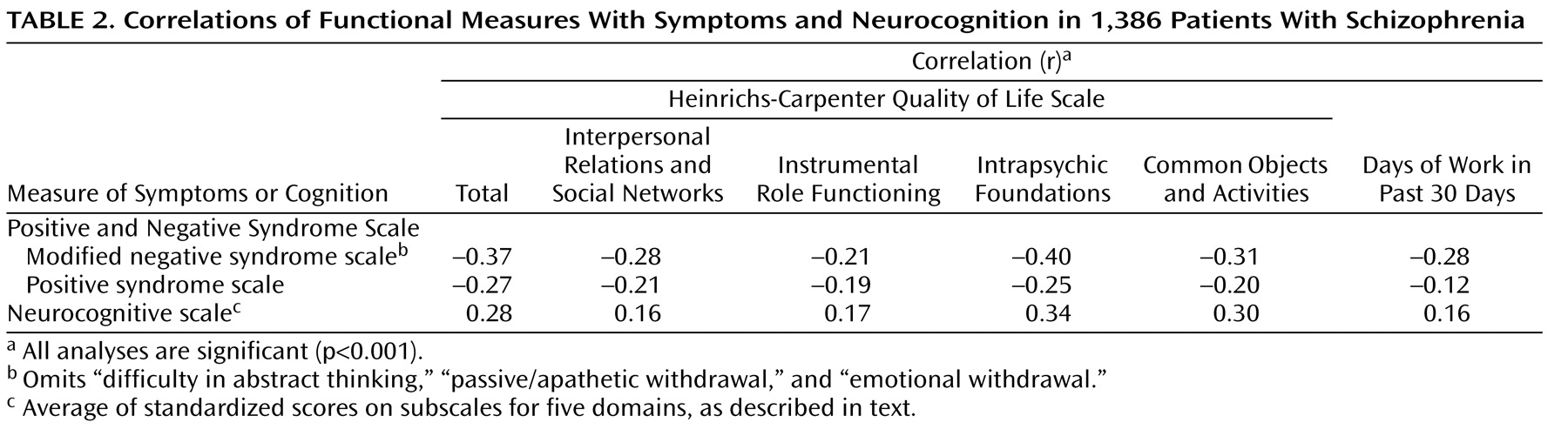
Bivariate analyses of correlations of the PANSS subscale scores and the neurocognitive score with the measures of community functioning showed significant relationships between functioning and measures of positive symptoms, negative symptoms, and neurocognition, with weak to moderate strength of association (
Table 2 ). The direction of the association between symptoms and functioning was negative (more severe symptoms were associated with poorer functioning), while the direction of the association between cognition and functioning was positive (better cognition was associated with better functioning). The Quality of Life Scale total score was more strongly correlated with negative symptoms than with either the neurocognitive score (Fisher z-transform, p=0.05) or the score for positive symptoms (p=0.03).
For the Quality of Life Scale subscales for interpersonal relations and instrumental role functioning and for the number of days of employment in the previous 30 days, the strengths of the correlations with positive symptoms and neurocognition were not significantly different in the Fisher z-transform test. The two other quality of life subscales, intrapsychic foundations and common objects and activities, were more strongly associated with neurocognition than with positive symptoms (p=0.05 and p=0.03, respectively).
Multivariate Analyses
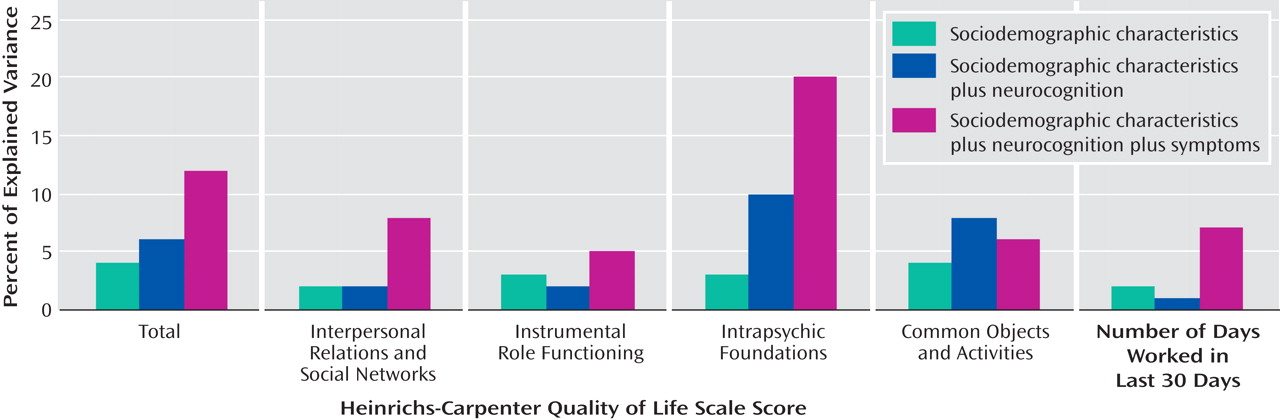
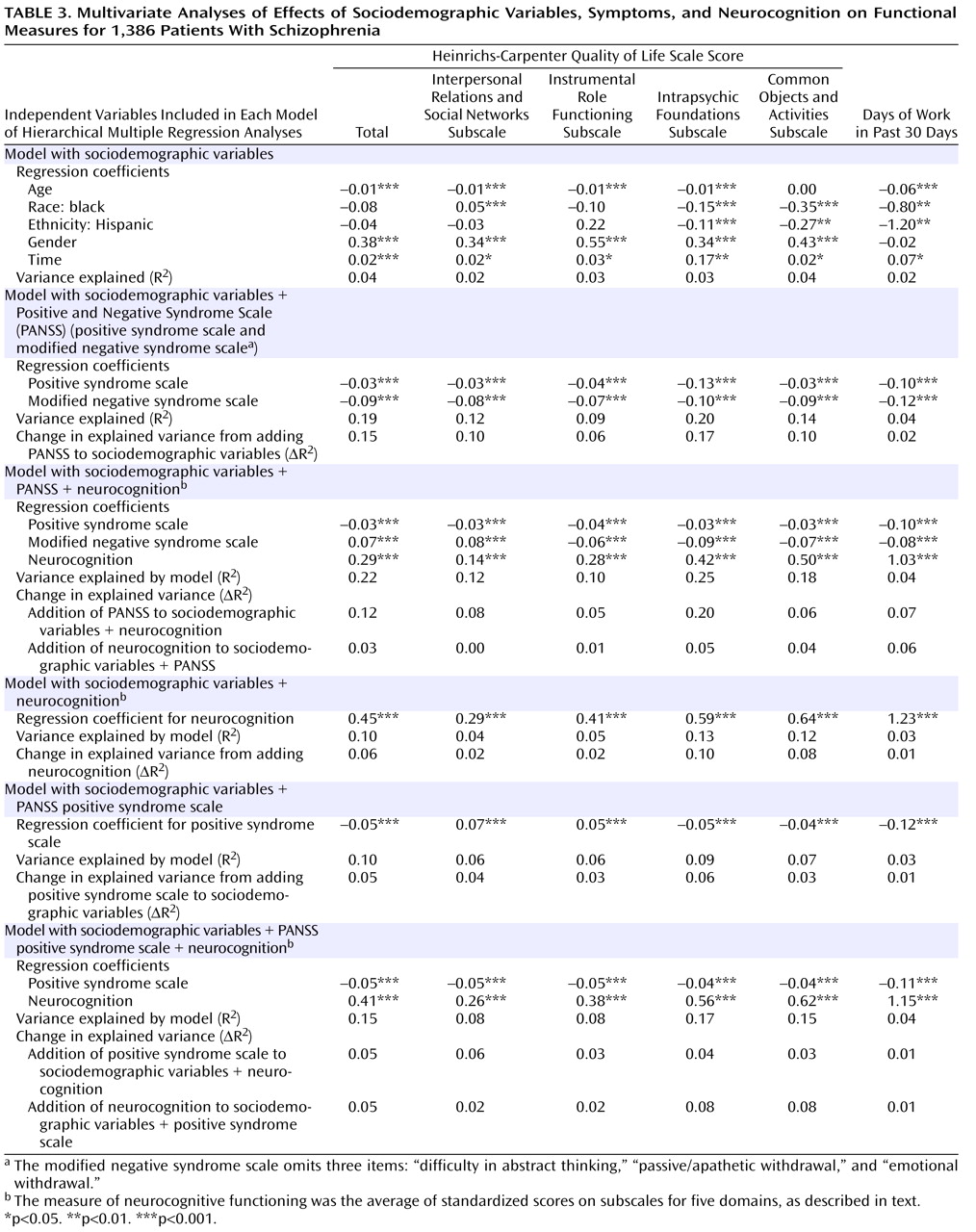
For economy of presentation, only the final step in each sequential model of the standard hierarchical multiple regression analyses is presented (
Table 3 ). Sociodemographic characteristics explained 4% of the variation in the total score on the Quality of Life Scale when entered on the first step, with a range of 2% to 4% on the quality of life subscales and days of employment (
Table 3, top panel;
Figure 1 ).
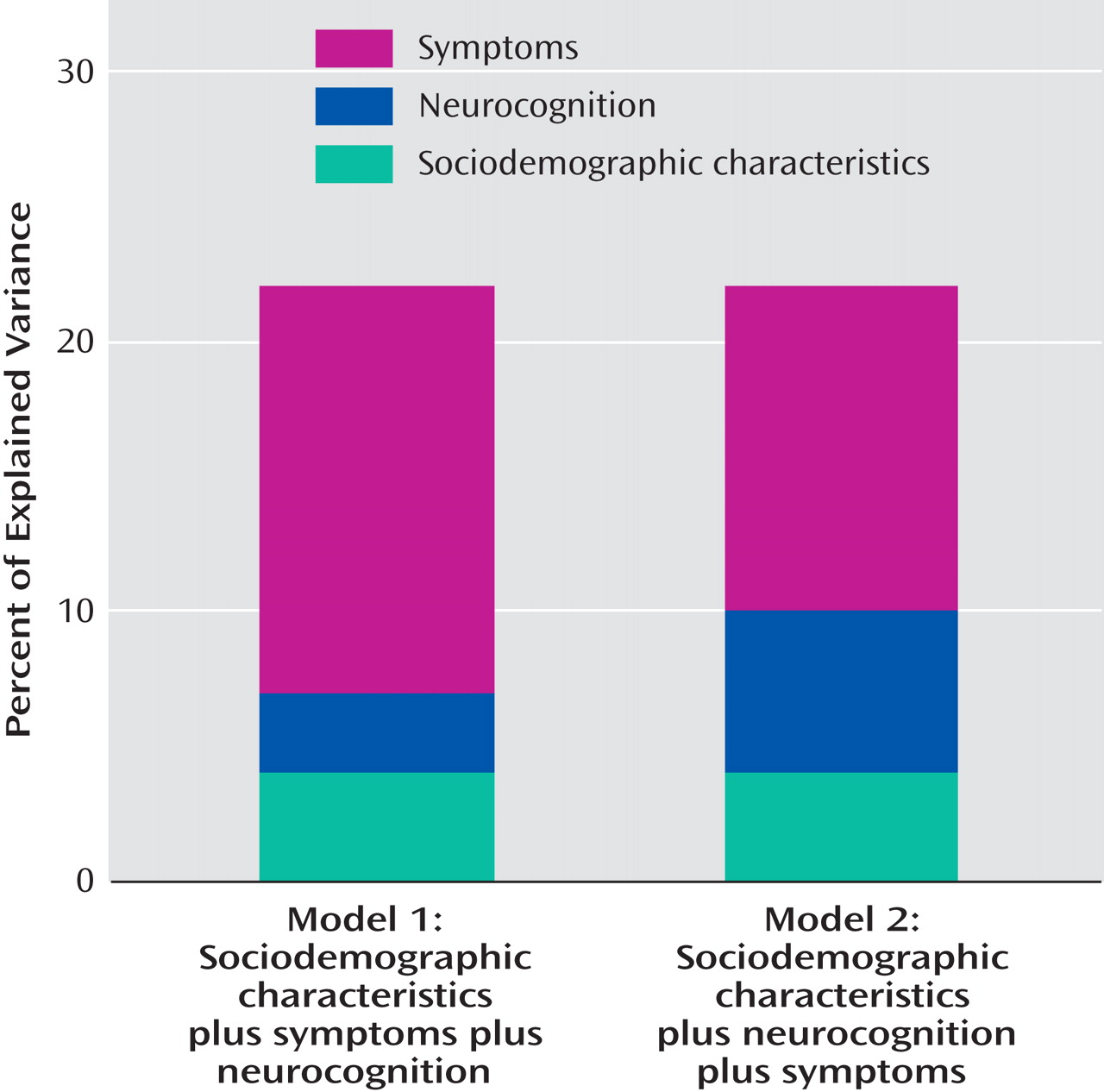
The addition of symptoms to sociodemographic characteristics increased the explained variance in the quality of life total score by 15% (
Figure 2 ), with changes in the R
2 value ranging from 2% to 17% (
Table 3, second panel). The PANSS modified negative syndrome scale was most strongly and negatively associated with the number of days worked in the previous 30 days, while the positive syndrome scale was most strongly and negatively associated with the intrapsychic foundations subscale of the Quality of Life Scale.
When neurocognition was added to both sociodemographic characteristics and symptoms, it explained an additional 3% of the variance in the Quality of Life Scale total score (
Figure 2 ), with the largest incremental explained variance for the number of days of employment in the previous 30 days and the least incremental explained variance in the quality of life subscale for interpersonal relations and social networks (
Table 3, third panel). The additional variance explained by neurocognition over and above symptoms (3%–6%) was less than half the variance explained by the addition of symptoms to neurocognition—12% for the Quality of Life Scale total score, with a range from 5% to 20%.
When neurocognition was added to the model including sociodemographic variables but not symptoms, it explained an additional 6% of the variance in the Quality of Life Scale total score (
Figure 2 ), with a range from 1% to 10% (
Table 3, fourth panel;
Figure 1 ). Thus, neurocognition added less than half as much to the explained variance as symptoms both when these measures were added to the model including sociodemographic variables alone and when neurocognition was added to the model that already included the other measure (i.e., when neurocognition was added to symptoms and when symptoms were added to neurocognition) (
Figure 2 ).
A more conservative replication of these analyses included only the positive syndrome scale as a symptom measure in addition to sociodemographic characteristics (i.e., the modified negative syndrome scale was excluded). After the positive syndrome subscale was added to sociodemographic variables (
Table 3, fifth panel), the model explained 10% of the variance in the Quality of Life Scale total score (ΔR
2 =5%, with an incremental range of 1%–6% on the other measures). The incremental effect of the positive syndrome subscale was not significantly different from the effect of adding neurocognition to sociodemographic variables on the total score, on the number of days of employment, or on two of the quality of life subscales (
Table 3, sixth panel).
Mixed-Model Regression Analysis
In the mixed-model analysis of the association of symptoms and neurocognition with the total Quality of Life Scale score, significant relationships were noted between quality of life and positive symptoms (r=–0.03, df=852, p<0.001), the modified negative symptom subscale (r=–0.06, df=852, p<0.0001), and neurocognition (r=0.30, df=852, p<0.0001), thus demonstrating independent effects for both symptoms and neurocognition in association with the measures of functioning (online data supplement Table 1).
The three independent variables representing positive symptoms, negative symptoms, and neurocognition also remained significantly related to the quality of life total score when these analyses were repeated 1) separately for each drug by using observations from phase 1 of the trial (there were only 22 observations for clozapine, but the nonsignificant associations were in the same directions as for the other drugs), 2) by using each of the five domains of the neurocognitive battery in separate analyses, 3) by limiting the analysis to baseline observations, and 4) by including adjustment for site effects and the potentially confounding effects of insight, depression, and extrapyramidal side effects (data supplement Table 1).
In the mixed-model analysis of the association of change in symptoms and neurocognition and change in quality of life, significant relationships were also noted between the changes in quality of life and positive symptoms (beta=–0.02, df=54, p<0.008), negative symptoms (beta=–0.05, df=54, p<0.0001), and neurocognition (beta=0.32, df=54, p<0.0001) (data supplement Table 2). The strengths of associations (magnitudes of coefficients) between change in the two symptom measures and change in quality of life were somewhat smaller in the longitudinal analysis than in the cross-sectional analysis (data supplement Table 1), but the strength of association was about the same for neurocognition. Because the numbers of observations available for individual drugs in phase 1 were small (range=52–135; see data supplement Table 2), most of these associations were not statistically significant, although the direction of the associations was consistent with the overall longitudinal analysis for 13 of 15 coefficients. The associations of change in symptoms and specific neurocognitive domains were all significant and in the same direction as those in the main longitudinal analysis, with little variation across domains. The result of the analysis that adjusted for site and additional clinical covariates was similar in magnitude to the outcome of the overall change analysis (last row in data supplement Table 2), although the symptom effects were larger and closer in magnitude to those observed in the mixed-model analyses of cross-sectional data (data supplement Table 1).
Discussion
This study examined the relationship of both neurocognition and schizophrenia symptoms to social and vocational functioning at a macrosocial level
(31), using the Heinrichs-Carpenter Quality of Life Scale and reported days of employment in a large group of patients (N=1,386). We used a newly constructed modification of the PANSS negative syndrome scale that eliminated items most directly overlapping with measures of community functioning, so as to avoid artificial inflation of the association of negative symptoms and functioning. Both symptoms and cognition, including positive symptoms considered alone, had statistically significant associations with the Quality of Life Scale total score, scores on its subscales, and a measure of recent employment; symptoms had significantly stronger associations on some measures. We also examined whether symptom severity would predict social functioning, independently of neurocognition, in hierarchical multiple regression analysis. In several of these analyses, symptoms contributed more to the incremental proportion of explained variance in the level of social and vocational functioning than measures of neurocognitive functioning, when each measure was added to models that included basic sociodemographic characteristics. Positive and negative symptoms together contributed an additional 15% to the explained variance in quality of life, after sociodemographic characteristics were controlled for, while neurocognition added an additional 6%. When entered after sociodemographic variables and symptoms, measures of neurocognitive functioning explained less incremental variance (3%) than was explained when symptoms were added to sociodemographic variables and neurocognition (12%). When we conducted a more conservative analysis using only the positive syndrome scale in addition to sociodemographic variables, positive symptoms explained an additional 5% of the variance in the quality of life total score, similar to the 6% additional explained variance when neurocognition was added to sociodemographic variables.
Neurocognition and both symptom measures were independently and significantly associated with quality of life in a mixed-model regression analysis. The findings were robust in analyses conducted separately for each drug, for each of the five domains of the neurocognitive battery, with the analysis limited to baseline observations, and with adjustment for site effects and the potentially confounding effects of insight, depression, and extrapyramidal side effects. The patterns observed in cross-sectional analyses were also replicated in a set of longitudinal analyses of the relationship between change in symptom measures and neurocognition and change in the Quality of Life Scale total score. The magnitudes of the symptom effects seemed somewhat smaller than in the cross-sectional analyses, but in a final analysis that controlled for insight, depression, and site effects, the magnitudes of effect were similar, confirming the previous results. Although this study is unique in addressing longitudinal associations, the results generally replicated the findings of an earlier study that used a similar analytic approach to data from patients who participated in a VA Cooperative Study
(12), although the magnitudes of the increments in the R
2 values were somewhat more modest in this study. Green, however, has noted that even small but statistically significant effects may be of potential clinical importance
(32) . The findings of this study thus confirmed our hypothesis that social or occupational functioning is significantly and independently associated with measures of both neurocognition and symptoms.
“Macrosocial” outcome measures such as the Quality of Life Scale evaluate relatively complex aspects of functioning, such as empathic ability, daily social interaction, and employment, and are contrasted with “microsocial” measures, such as tests of interpersonal and social problem solving, that address more isolated skills in a laboratory context rather than in the context of community living
(31) . While microsocial measures assess subcomponents of general functioning, such as social information processing, and are more similar in form and focus to tests of neurocognition, macrosocial measures address real-world functions, such as living in independent housing, having frequent social interactions, and employment, and are thus more likely to be associated with general psychopathology. Both are important in the study of outcomes of schizophrenia.
While previous studies have examined the association of either neurocognition
(2) or psychopathology
(33) separately and demonstrated significant associations with various measures of functioning, studies that have not included both symptom and neurocognitive measures contemporaneously are limited because they do not address the substantial variance that is shared between symptoms and neurocognition. Such models assign all variance accounted for to the particular type of measure under study. The major advantage of the methods used here and previously
(12) is that we simultaneously examined the independent association of both kinds of measures with social functioning as well as their incremental association in sequential analyses.
Several other longitudinal studies have also found symptom measures, especially when they are assessed after initiation of treatment, to be as good at predicting future functioning as are measures of neurocognition
(34 –
36) . Consistent with our analyses, these longitudinal comparisons of cognitive and symptom measures in predicting functional outcomes support the notion that both kinds of measures play distinctive roles in predicting future functioning.
Other cross-sectional studies have also found significant correlations between symptoms and social and vocational functioning
(6,
7,
12,
37,
38) . In contrast to these studies, Velligan et al. presented evidence that the relationship of symptoms to daily functioning was largely attenuated in models including neurocognitive variables
(9) . However, their study group was limited to chronically ill, but recently admitted, inpatients. In contrast, our study, like several others
(6,
7,
12), primarily involved observations from outpatients residing in community settings. Residual symptoms among outpatients appear more likely to reflect enduring clinical traits associated with long-term functional status, as compared to the highly disorganized and transient states that emerge during acute exacerbations that require hospitalization.
The findings of this study that symptoms (negative and positive) and neurocognitive functioning are both independently significant correlates and predictors of social and vocational functioning have potentially important implications for treatment development. In recent years efforts have been made to develop both behavioral therapies and pharmacotherapies that are targeted at improving both symptoms and cognitive functioning. With the increased study of cognitive deficits in the last 10 years, “cognitive rehabilitation” interventions have been developed to directly address these deficits. Several reviews
(39) have concluded that cognitive rehabilitation approaches seem to be capable of improving cognitive performance, psychiatric symptoms, and everyday functioning, although it is not known how sustainable these gains can be. When coupled with psychosocial interventions such as supported employment, cognitive rehabilitation has been found to yield substantial synergistic improvements in employment outcomes
(40,
41) .
Another line of research has focused on cognitive-behavioral therapy (CBT) and has also shown significant benefits in reducing symptoms among some patients with schizophrenia
(42,
43) . While these studies differ on a number of dimensions—including duration of intervention, number of sessions, comparison treatment, and outcome measures—CBT seems to be particularly effective in ameliorating psychopathology, even among inpatients with acute psychotic episodes, with effect sizes as large as 0.65 and continued gains over time
(44) .
Recent studies have suggested that the cognitive improvement observed after treatment with atypical antipsychotics is consistent in magnitude with practice effects
(17), and some have questioned earlier findings suggesting neurocognitive advantages of the second-generation antipsychotics
(32) . Some of the emphasis on the development of pharmacotherapies for neurocognition seems to be based on the assumption that symptomatic treatments are generally adequate.
A limitation of this study that requires comment is that at many CATIE sites the rater who completed the PANSS also completed the Quality of Life Scale and at some of these sites a different rater administered the neurocognitive tests, thus introducing a potential rater’s bias that could have inflated the specific correlation between these two variables.
The results of the present reanalysis of CATIE data, nevertheless, suggest that efforts should continue on all fronts, behavioral and pharmacological, to address both symptoms and neurocognitive deficits. Both kinds of impairments, as we have seen, have independent adverse effects on community functioning, and perhaps the best development paradigm would move forward on both behavioral and pharmacological treatments that target both symptoms and neurocognition. It seems advisable to systematically test combinations of therapies in the hope that synergies and positive interactions may lead to new and more effective treatment strategies in addition to individual interventions.