One of the most consistent molecular findings from schizophrenia research is the dysfunction of specific subsets of gamma-aminobutyric acid (GABA) expressing interneurons
(1 –
4) . Reductions in mRNA and protein levels of glutamic acid decarboxylase 67 (GAD
67 ) have been found in specific interneurons of the prefrontal cortex
(5 –
7) and limbic system in individuals with schizophrenia
(8,
9), and similar findings have been observed in analyses of whole cerebellar tissue
(10,
11) . The same prefrontal cortex interneurons with GAD
67 reductions have also shown decreases in the presynaptic GABA plasma membrane transporter-1 (GAT-1)
(12) . Compensatory postsynaptic changes, such as increases in GABA type A (GABA
A )-α
2 receptor density and GABA
A receptor radioligand binding, in the prefrontal cortex and anterior cingulate cortex have also been reported
(13 –
15) . In addition, single nucleotide polymorphisms (SNPs) in the promoter region of the GAD
67 gene expression have been associated with gray matter reductions in patients with childhood onset schizophrenia
(16) and decreased cognitive functioning in adult schizophrenia
(17) . Given the role of GABAergic interneurons in the modulation of excitatory output, dysfunction in specific interneurons may mediate some of the positive, negative, and cognitive symptoms seen in schizophrenia.
Aside from its role in motor coordination, the cerebellum has been shown to contribute to cognitive functioning
(18) through connections of the posterior lateral hemispheres to the prefrontal cortex
(19) . Thus, physiological deficits in this brain region could contribute to the pathophysiology of schizophrenia. Supporting this hypothesis, neuroimaging studies have described increased blood flow and glucose consumption in the cerebellum among schizophrenia patients
(20 –
22) . Molecular studies have revealed decreased cerebellar expression of the GABAergic protein reelin and the GABA synthesizing enzymes GAD
67 and GAD
65 (10,
11) as well as increased expression of the axonal chemorepellent Semaphorin 3A
(23) and increased expression of granule cell-selective, activity-dependent, brain-derived neurotrophic factor, and growth-associated protein of 43 kDa (GAP-43) genes
(24) . To further explore the function of cerebellar interneurons in schizophrenia, we investigated changes in transcript levels of GABAergic markers,
N -methyl-
d -aspartate (NMDA) receptor subunits, and local circuit neuromodulators of GABA release using total RNA prepared from the posterior lateral cerebellar hemispheres of postmortem male schizophrenia subjects and age- and postmortem interval-matched male comparison subjects. Alterations in specific GABAergic transcripts point to a common pathophysiological mechanism across different brain regions and implicate the cerebellum in the cognitive and motor dysfunction observed in individuals with schizophrenia.
Results
GABAergic Marker Expression in the Lateral Cerebellar Hemisphere of Schizophrenia Subjects
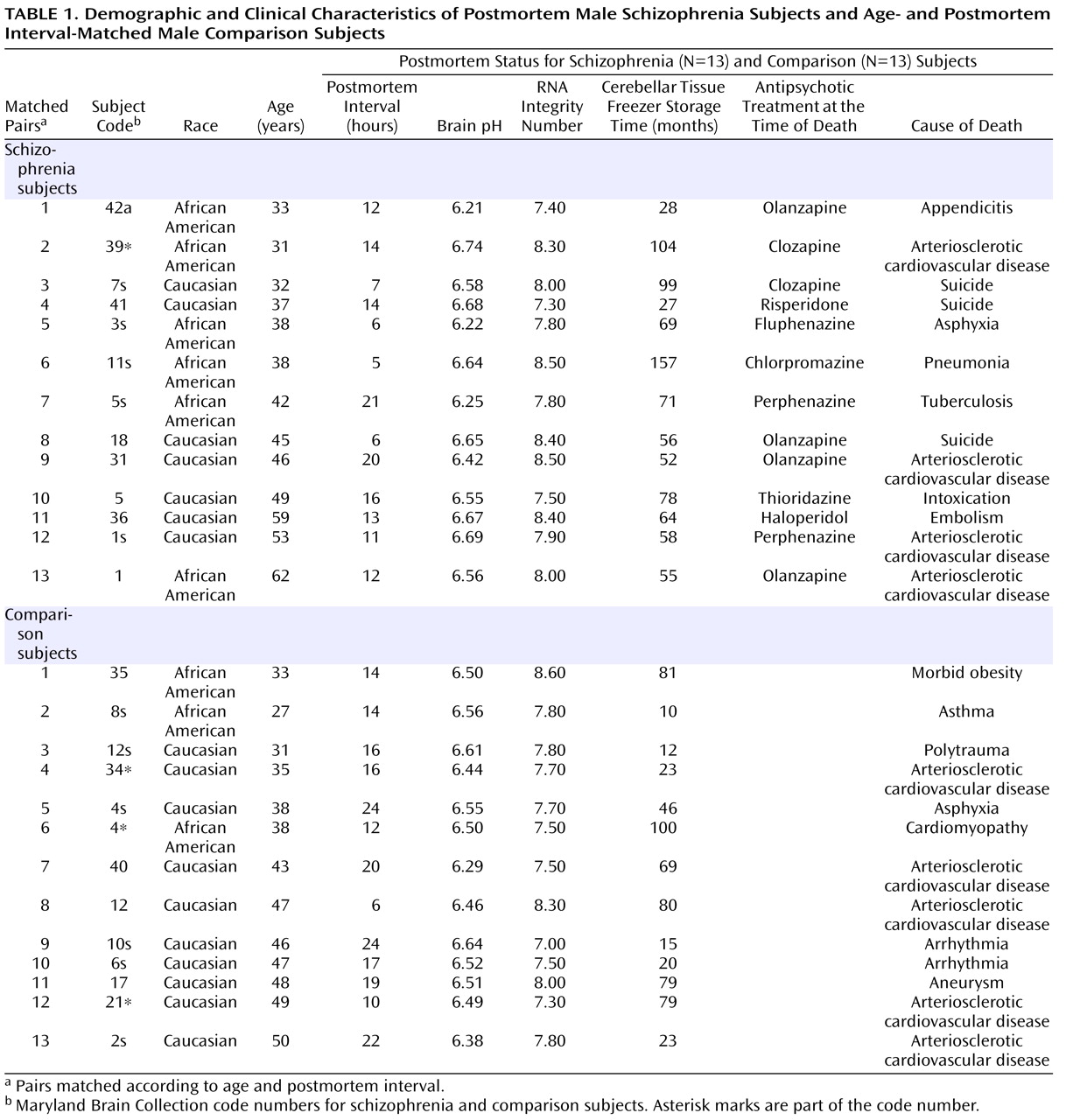
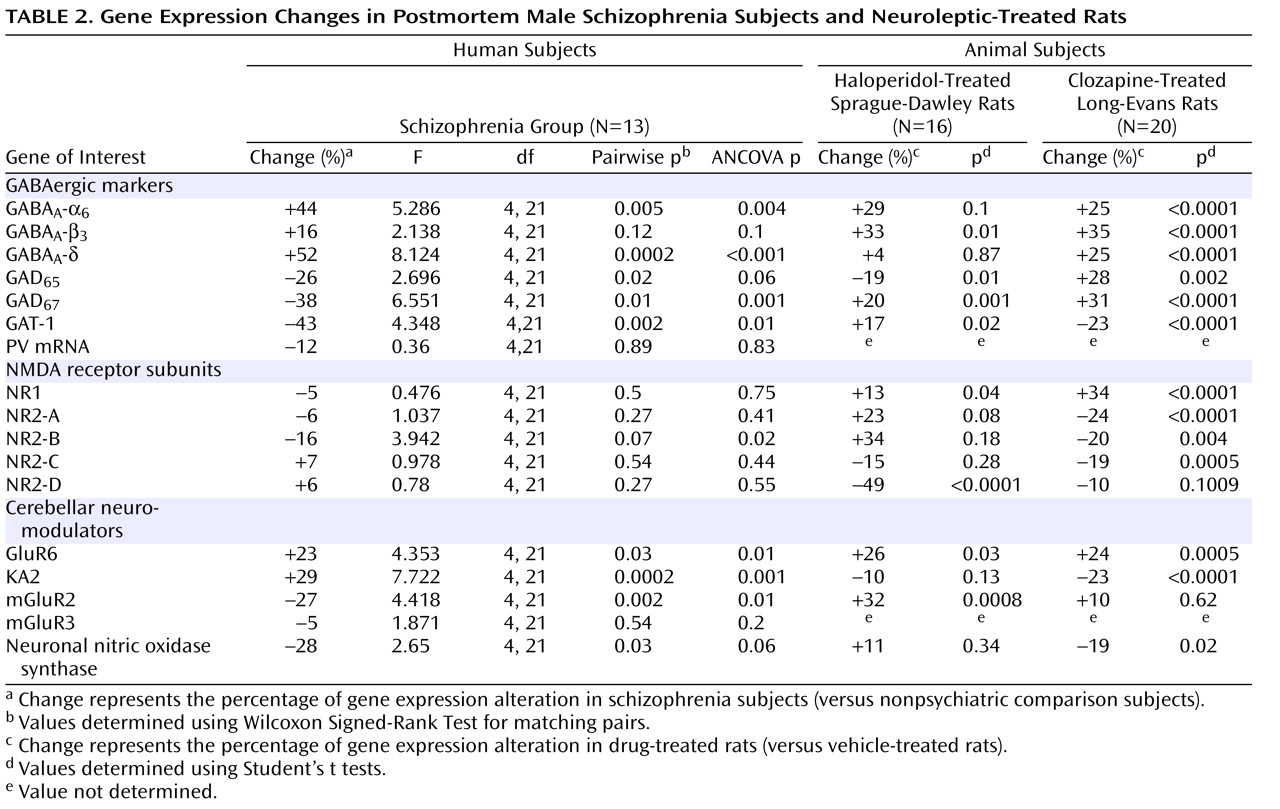
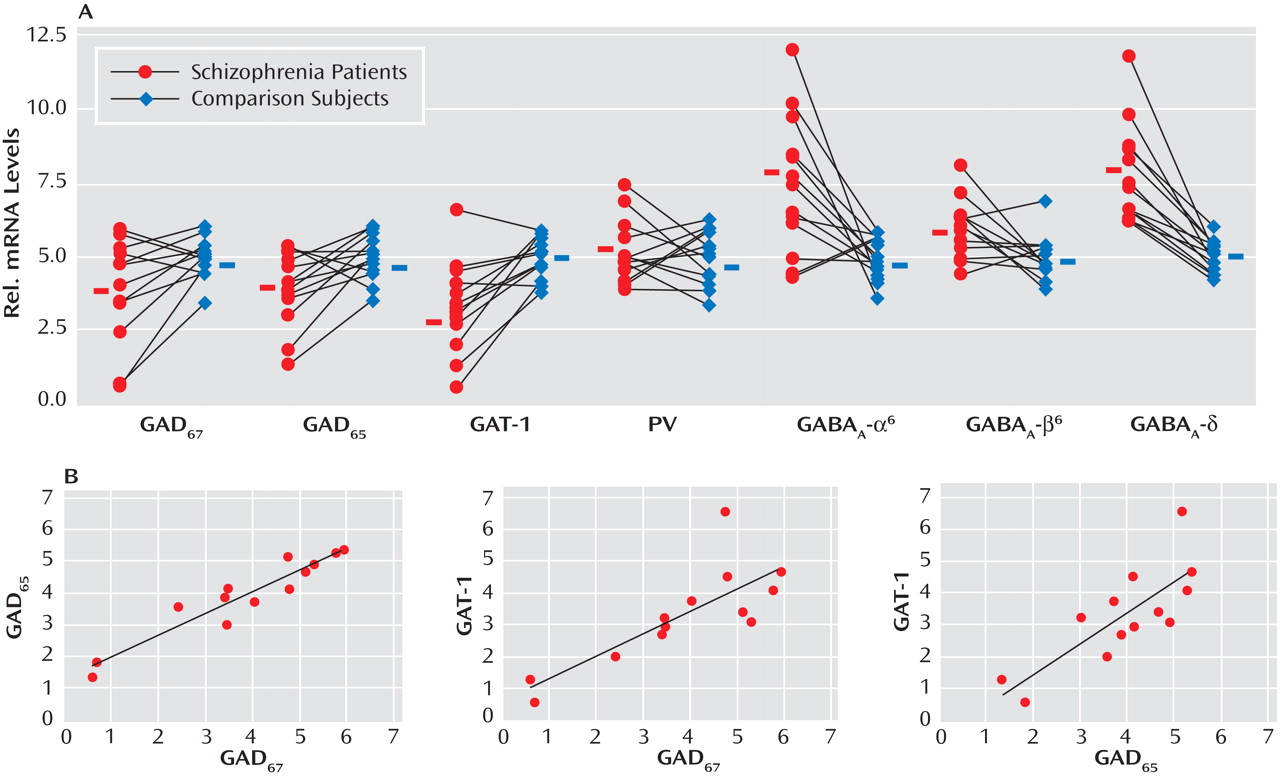
Given the evidence of GABA dysfunction among individuals with schizophrenia, we sought to characterize the condition of inhibitory interneurons in the cerebellum of schizophrenia subjects in order to 1) investigate the mechanisms underlying cerebellar abnormalities and 2) examine the nature and extent of GABA dysfunction in different neuronal populations. Initial studies examined the expression of seven markers of GABA function in the lateral cerebellar hemisphere of 13 pair-matched schizophrenia and comparison subjects using real-time PCR. To normalize the levels of a specific mRNA, β-actin was utilized because the expression of this particular mRNA did not differ between schizophrenia and comparison subjects (see the figure in the data supplement accompanying the online version of this article). Analysis of GABAergic transcript levels in schizophrenia subjects and matched comparison subjects using the Wilcoxon Signed-Rank Test revealed significant decreases in mRNA levels of GAD
67, GAD
65, and GAT-1 (schizophrenia to comparison group ratio=0.62, 0.74, and 0.57, respectively). However, no decreases in mRNA levels of parvalbumin were found (
Table 2,
Figure 1 ). Similar decreases in these transcripts were observed when cyclophilin was used as the housekeeping gene (see the data supplement accompanying the online version of this article). Subjects with schizophrenia also showed significant increases in mRNA levels for the granule-cell extrasynaptic GABA
A receptor subunits α
6 (schizophrenia to comparison group ratio=1.44) and δ (schizophrenia to comparison group ratio=1.53) relative to β-actin mRNA. However, no changes were found in GABA
A -β
3 mRNA, which is expressed in several cell types. In addition to pairwise analyses, we evaluated the effect of potential confounding variables, such as age, freezer storage time, and RNA integrity number, using ANCOVA. As detailed in
Table 2, ANCOVA results confirmed that mRNA levels of GAD
67, GAT-1, GABA
A -α
6, and GABA
A -δ were significantly different for schizophrenia subjects relative to comparison subjects (p<0.05), while mRNA levels of GAD
65 were close to significant (p=0.06).
To examine possible gene-gene interactions, mRNA levels were analyzed using Spearman’s r correlations. In the schizophrenia group, positive correlations were 1) GAD
67 with GAD
65 (r=0.922, p<0.001) and 2) GAT-1 (r=0.797, p=0.001) and GAD
65 with GAT-1 (r=0.770, p=0.002) (
Figure 1 ). Comparison subjects also showed a positive correlation between GAD
67 and GAT-1 (r=0.646, p=0.02).
NMDA Receptor Subunit Expression in the Lateral Cerebellar Hemisphere of Schizophrenia Subjects
In addition to GABA deficits, NMDA dysfunction has been found in schizophrenia patients
(28) . In the present study, analyses of the levels of NMDA receptor subunits NR1, NR2-A, NR2-B, NR2-C, and NR2-D, using the Wilcoxon Signed-Rank Test, revealed no significant changes except for a tendency toward decrease in the Golgi cell-selective subunit NR2-B
(29) (schizophrenia to comparison group ratio=0.84, p=0.07 [
Table 2 ]). Furthermore, when considering the effect of age, freezer storage time, and RNA integrity number using ANCOVA, we found a significant decrease in the levels of NR2-B among schizophrenia subjects (p=0.004 [
Table 2 ]). In addition, the levels of NR2-B in the schizophrenia group were positively correlated with the levels of NR1 (r=0.553, p=0.05), NR2-A (r=0.553, p=0.05), and NR2-D (r=0.663, p=0.01).
Cerebellar Neuromodulator Expression in the Lateral Cerebellar Hemisphere of Schizophrenia Subjects
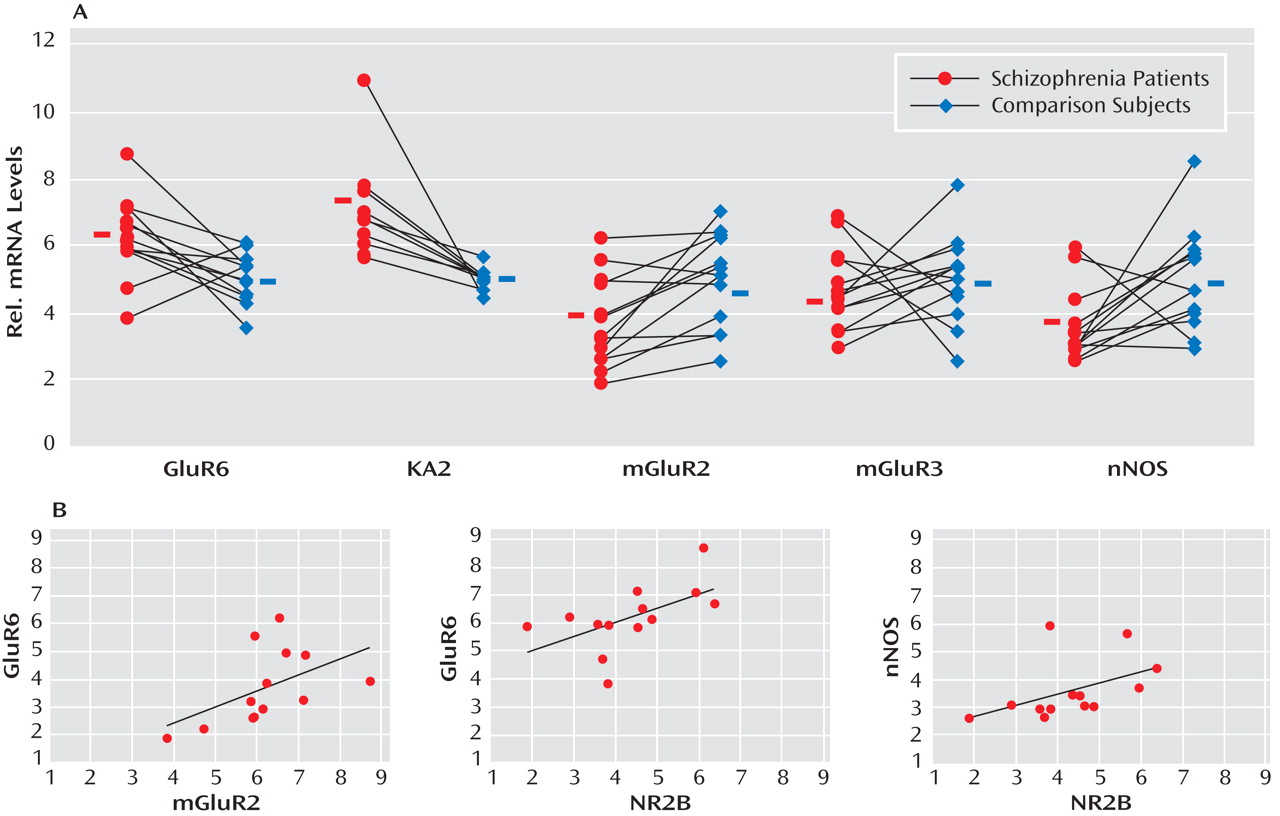
GABA release onto cerebellar granule cells is regulated at the cerebellar glomerulus, where pontine mossy fibers and Golgi cells synapse onto granule-cell dendrites in a close structural and functional unit. Thus, we examined the major modulatory factors of this unit, which could contribute to decreased GABA neurotransmission to granule cells. Of these modulatory factors, we observed significant decreases in GABAergic transcript levels of neuronal nitric oxide synthase (schizophrenia to comparison group ratio=0.72), present in granule cells and molecular layer interneurons
(30), and the Golgi cell-specific mGluR2
(31) (schizophrenia to comparison group ratio=0.73) but not mGluR3 (
Figure 2 ). Interestingly, both nitric oxide production by neuronal nitric oxide synthase and presynaptic mGluR2 receptor activation modulate GABA release from Golgi cells
(32) . Moreover, we found significantly increased levels of the glutamatergic kainate receptor subunits GluR6 (schizophrenia to comparison group ratio=1.23) and the granule cell-specific subunit KA2 (schizophrenia to comparison group ratio=1.29
[33] ). ANCOVA revealed that all changes in GABAergic transcript levels were significant (p<0.05) except for changes in neuronal nitric oxide synthase, which were near significant (p=0.06 [
Table 2 ]).
Correlations of Transcripts Levels in Golgi Cells, Granule Cells, and Local Cerebellar Circuitry Components
To further investigate the functional integrity of the cerebellar glomerulus in schizophrenia subjects, interactions between specific markers in the Golgi-granule cell circuit were analyzed. We found positive correlations for the Golgi cell-selective NR2-B with GAD 67 (r=0.922, p<0.001), GAD 65 (r=0.854, p<0.001), and GAT-1 (r=0.671, p=0.01) and for mGluR2 with GAD 65 (r=0.575, p=0.04), all of which were decreased in the schizophrenia group. In addition, GABAergic transcripts expressed in granule cells showed correlations for NR2-C with GABA A -α 6 (r=0.639, p=0.02), GABA A -β 3 (r=0.722, p=0.005), GABA A -δ (r=0.652, p=0.02), and KA2 (r=0.726, p=0.005), which were all increased among schizophrenia subjects. Finally, we found that neuronal nitric oxide synthase correlated with GABA A -β 3 (r=0.757, p=0.003); GABA A -α 6 correlated with GluR6 (r=0.659, p=0.01); and KA2 correlated with GABA A -δ (r=0.600, p=0.03).
Positive correlations for components of Golgi cell-granule cell circuitry were 1) NR2-B with GABA A -β 3 (r=0.600, p=0.03), GluR6 (r=0.594, p=0.03), and neuronal nitric oxide synthase (r=0.624, p=0.02) and 2) mGluR2 with GluR6 (r=0.713, p=0.006) and GABA A -α 6 (r=0.797, p<0.001). Significant negative correlations were observed for GAT-1 with NR2-C (r=–0.553, p=0.05) and KA2 (r=–0.779, p=0.002), presumably reflecting a compensatory mechanism to increase GABA neurotransmission in the synaptic cleft.
Medication Effects on Gene Expression in Haloperidol- and Clozapine-Treated Rats
Both schizophrenia and comparison subjects were receiving antipsychotics at the time of death, with approximately one-half of the schizophrenia group being treated with typical antipsychotics and one-half being treated with atypical antipsychotics (
Table 1 ). Analysis of gene expression levels between schizophrenia subjects who were receiving atypical versus typical antipsychotics showed a significant increase in GAD
67 gene expression for the former (p=0.03 [Mann-Whitney Rank Sum Test]). This finding suggests that medication type may have an effect on gene expression levels in individuals with schizophrenia. Therefore, we analyzed the levels of the same mRNAs in the cerebellum of rats chronically treated with approximately 1 mg/kg/day of the typical neuroleptic haloperidol. As detailed in
Table 2, we found significant increases in GABA
A -β
3, GAD
67, GAT-1, NR1, GluR6, and mGluR2 in these rats. However, a significant decrease was seen in the expression of GAD
65 and NR2-D transcripts.
To examine the effects of atypical antipsychotics, rats were treated with clozapine for 21 days (10 mg/kg/day). As detailed in
Table 2, these animals expressed increased mRNA levels of GABA
A -β
3, GAD
67, and GAD
65, while GAT-1 expression was significantly decreased. The NMDA receptor subunit NR1 was significantly increased, whereas NR2-A, NR2-B, and NR2-C were all decreased. Finally, GluR6 was significantly increased, KA2 and neuronal nitric oxide synthase were decreased by clozapine treatment. Overall, our results indicate that clozapine could compensate, in part, for the decreased level of GABA synthesizing enzymes and other gene expression changes observed in schizophrenia subjects.
Discussion
An increasing number of studies support the hypothesis that GABA neurotransmission is deficient in individuals with schizophrenia
(2 –
4) . Although GABAergic deficits have been well characterized in the prefrontal cortex
(5 –
7) and limbic system
(8,
9), few studies have examined these deficits in the cerebellum
(10,
11) . The present study demonstrated GABA transmission deficits in the lateral cerebellar hemisphere in individuals with schizophrenia, which implicates selective impairment in local granule-cell Golgi-cell communication, along with altered expression of neuromodulators of granule cell activity. Disinhibition of granule cells by deficient tonic inhibition from Golgi cells could disrupt cerebellar output by Purkinje cells, which in turn may affect other brain regions in the cortico-cerebellar-thalamic-cortical circuit, including the prefrontal cortex
(34) .
Several discrete GABAergic interneurons are located in the cerebellum, most notably Golgi cells in the granule layer and basket and stellate cells in the molecular layer. Although basket and stellate cells provide feed-forward inhibition from granule cells to Purkinje cells, Golgi cells provide the sole source of feedback inhibition to granule cells
(35) . All of these interneurons express the GABA synthesizing enzymes GAD
65 and GAD
67 . Our findings indicate that the levels of these GABAergic transcripts are decreased in individuals with schizophrenia relative to comparison subjects, suggesting that GABA synthesis may be impaired in schizophrenia patients. Consistent with our findings, Fatemi et al.
(11) reported decreases in protein levels of both GAD
65 and GAD
67 in the cerebellum of a separate cohort of schizophrenia subjects.
Although GABA synthesis cannot be directly measured in postmortem tissue, other markers can be investigated to infer whether GABAergic transmission is reduced. For example, decreased levels of the presynaptic GAT-1 may reflect a compensatory attempt to increase synaptic GABA. Mature Golgi and basket/stellate cells express GAT-1, while Purkinje cells and glia do not
(36), suggesting that GAT-1 deficits may be restricted to specific GABAergic interneurons. This is consistent with a similar decrease in GAT-1 observed in the prefrontal cortex of schizophrenia patients in association with GAD
67 deficits
(12) . Interestingly, the calcium binding protein parvalbumin, which has been found to be decreased in the prefrontal cortex in individuals with schizophrenia
(6), was not decreased in the present study, suggesting that different subtypes of interneurons may be affected in the cerebellum. Parvalbumin is expressed primarily in Purkinje and basket/stellate cells but only in a small subpopulation of Golgi cells
(37) . Conversely, the significant increases in the expression of the extrasynaptic GABA
A receptor subunits α
6 and d suggest that a compensatory mechanism may occur in order to increase GABA receptivity by granule cells. As a result of freezing the tissue samples, we were unable to confirm the localization of specific transcripts by in situ hybridization. However, our findings point to Golgi cells as the predominately affected cerebellar interneuron in schizophrenia.
A competing, but not mutually exclusive, hypothesis for schizophrenia proposes that NMDA receptor-mediated neurotransmission may be deficient in individuals with schizophrenia
(28) and that GABAergic interneurons are particularly vulnerable to NMDA receptor hypofunction
(38) . In the present study, GABAergic interneuron function in the cerebellum was further investigated by measuring the expression of cell-selective NMDA receptor subunits. Although each cell type expresses the obligatory NR1 subunit, Purkinje cells express primarily NR2-A; Golgi cells express primarily NR2-B; granule cells express primarily NR2-C; and basket/stellate cells express primarily NR2-D
(29) . Among these subunits, we found a decrease in the levels of the Golgi cell-selective NR2-B. The strong correlations between NR2-B expression and GAD
67, GAD
65, and GAT-1 expression in the schizophrenia group further suggests that GABA transmission deficits may be localized to Golgi cells. Alternatively, it is possible that the number of Golgi cells and not their activity may be decreased in individuals with schizophrenia.
GABA release by Golgi cells is regulated by various factors in both Golgi and granule cells, including Golgi cell-specific mGluR2
(31) and the granule cell-located neuronal nitric oxide synthase
(32) . Activation of both systems normally decreases GABA release by Golgi cells, and both were decreased in our cohort of schizophrenia subjects (
Figure 2 ), presumably compensating for decreased levels of GABA in the synapse. On the other hand, mGluR3, which is expressed in Golgi cells and glia
(31), was not altered in the schizophrenia group. In addition, although decreased levels of mRNA in the receptor subunits GluR6 and KA2 have been previously reported in the prefrontal cortex and hippocampus of schizophrenia patients
(33,
39), we observed increased cerebellar expression of these kainate receptors. Since both markers are expressed in granule cells and these cells have shown increased expression of activity dependent-genes in the same group of schizophrenia subjects
(24), it is possible that the changes in kainate receptor subunits may reflect a change in basal granule cell activity.
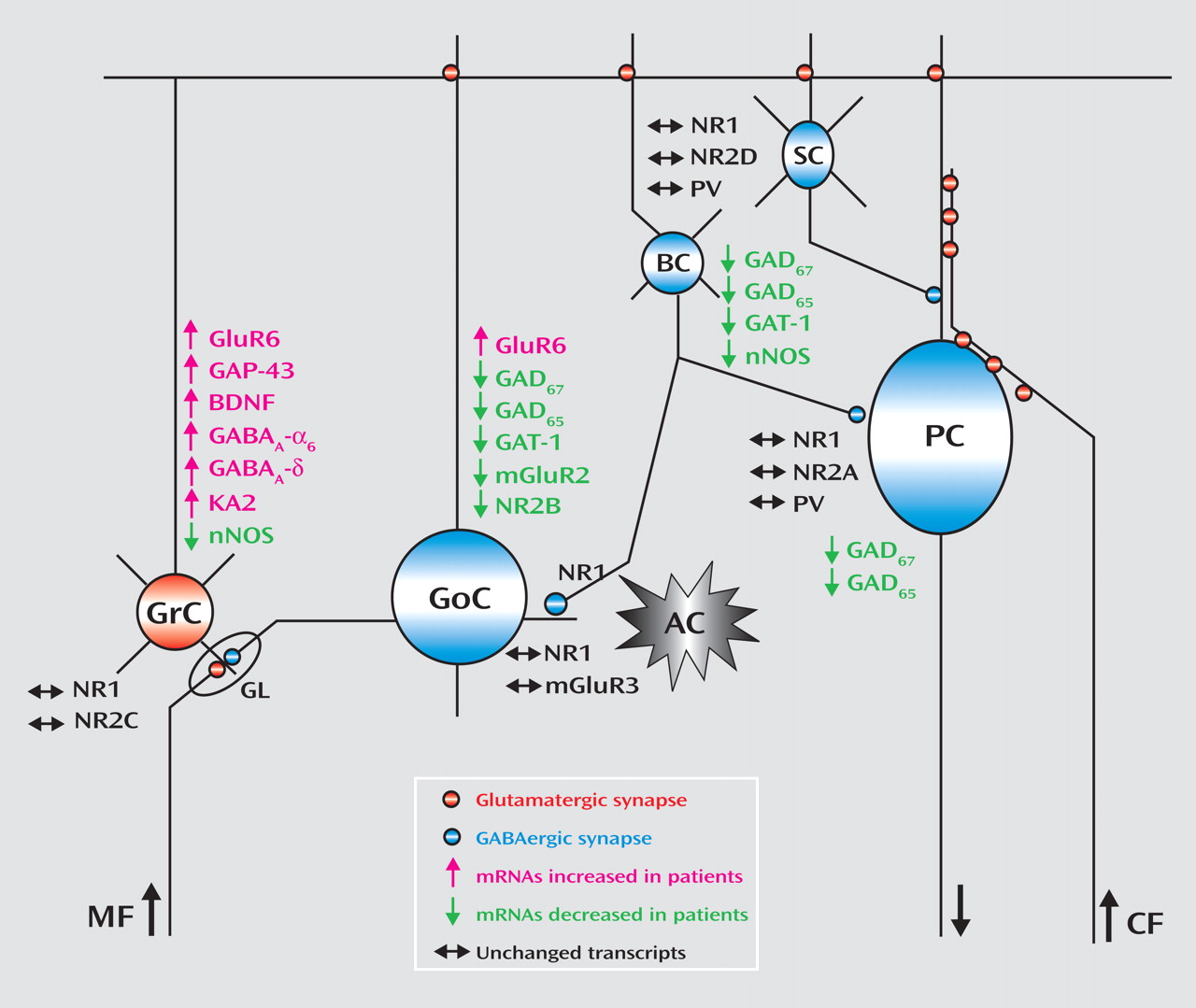
The changes in gene expression and putative cerebellar microcircuit alterations are illustrated in
Figure 3 . Although postmortem human tissue studies only provide a limited view of the premortem state and the use of tissue homogenates does not allow localization of transcripts to specific cell types, analyses of gene expression patterns using gene-gene correlations are valuable tools to infer the functional relevance of the observed changes across different cells in the network. An alteration in GABA neurotransmission between Golgi and granule cells is further evidenced by the positive correlations between the modulators of GABA release neuronal nitric oxide synthase and mGluR2 and between the Golgi specific marker NR2-B and neuronal nitric oxide synthase. Moreover, the negative correlations for GAT-1 with the granule cell-specific receptor subunits NR2-C and KA2 suggest a defect in modulation of GABA release by Golgi cells and increased granule cell firing. Although these findings are intriguing, animal models will need to be developed in order to confirm the dynamic nature of the interactions between granule cell and Golgi cell gene-expression patterns reported in the present study.
Our gene expression findings in schizophrenia subjects point to dysfunctional regulation of GABA release between Golgi and granule cells but do not take into account any effects of antipsychotic medication. Although antipsychotics have been shown to affect gene expression in several brain regions, these alterations have not been demonstrated in the cerebellum. As detailed in
Table 2, haloperidol treatment resulted in increased levels of GAD
67, mGluR2, NR2-B, GAT-1, and neuronal nitric oxide synthase in chronically treated rats, whereas clozapine was more effective in increasing the expression of GAD
67 and GAD
65 . Comparison of the levels of GluR6, GABA
A -α
6, and GABA
A -δ receptor mRNAs in schizophrenia subjects and treated animals suggests that the observed increases of these transcripts in the schizophrenia group could be explained by medication effects. In contrast, the increases in KA2 mRNA seen in the schizophrenia group could not be attributable to antipsychotic treatment, since both haloperidol and clozapine decreased the levels of KA2 mRNA. In addition, medication effects could account for an underrepresentation of the changes in NR2-C and NR2-D gene expression in the schizophrenia group. Based on the results of the chronic neuroleptic exposure in rats, haloperidol seems to have a more beneficial effect on gene expression in the local cerebellar circuit relative to clozapine. Furthermore, although both medications were effective in increasing GAD
67 expression in rats, neither treatment was effective in schizophrenia subjects. Our results support the idea that combining different types of antipsychotics with GABA
A receptor agonists may provide enhanced benefits for treating the underlying GABA-glutamate pathophysiology of schizophrenia.
In summary, we found that the expression of multiple GABAergic markers is decreased in the lateral cerebellar hemisphere among individuals with schizophrenia and this deficit is most likely localized to Golgi cells. Overall, patient mRNA expression data suggest that GABA neurotransmission deficits could be responsible for increased granule cell activity and disinhibition of glutamatergic signaling in the cerebellum, leading to aberrant cerebellar output. These changes may underlie not only the alterations in cerebellar blood flow observed in our schizophrenia cohort, but also, and most importantly, the clinical symptoms associated with abnormal cerebellar function in schizophrenia
(40) .