Recently, assessments of brain functional connectivity were conducted in order to investigate the level of integration of brain systems at a resting state when no task was performed
(20) . Low-frequency (0.01–0.8 Hz) fluctuations of the blood-oxygen-level-dependent (BOLD) signal in the resting state are considered to be physiologically meaningful and related to spontaneous neural activity
(21) . Although fMRI studies can assess disturbances in functional connectivity when patients perform a particular task, assessment of resting state connectivity may have different and potentially broader significance. Abnormal bilateral fronto-parietal, fronto-cingulate, and fronto-thalamic connectivity have been observed in schizophrenia patients during the resting state
(22,
23) . However, it is unclear whether these connectivity alterations were associated with morphometric deficits or clinical symptom severity. Until recently, very few studies investigated whether gray matter deficits in schizophrenia affect cerebral functional networks, and no study, to our knowledge, has determined whether symptoms of schizophrenia are associated with the functional connectivity of areas with gray matter deficits.
Discussion
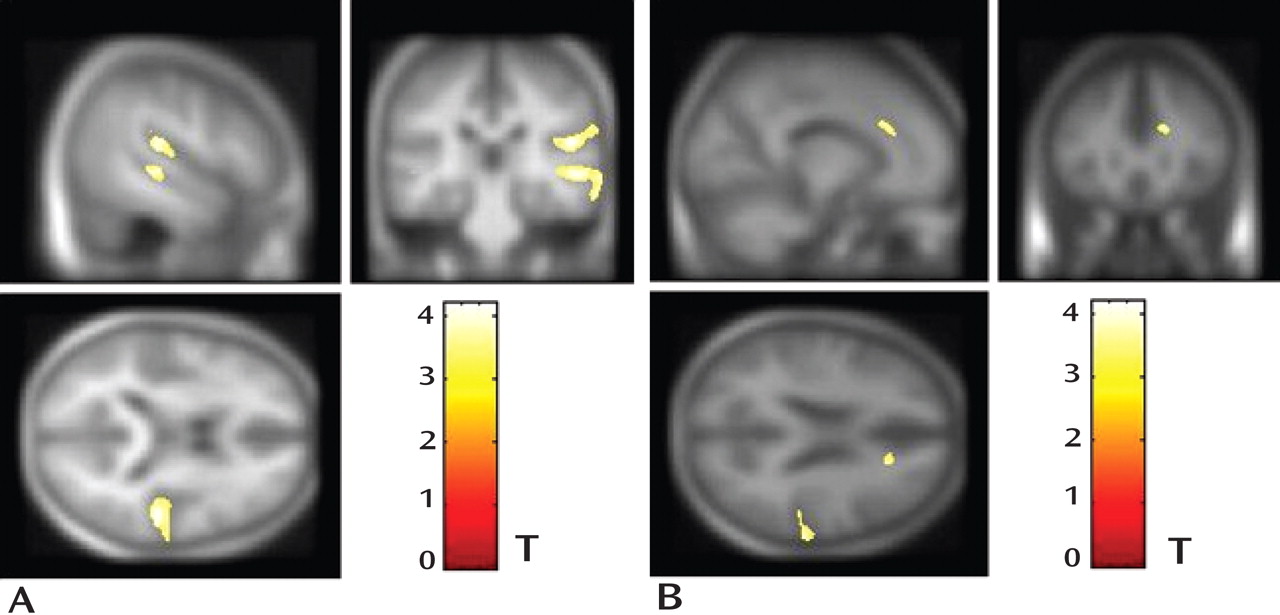
The present study examined gray matter abnormalities in a relatively large sample of antipsychotic-naive first-episode schizophrenia patients. Decreases in gray matter volume were observed in the right anterior cingulate gyrus, right middle temporal gyrus, and right superior temporal gyrus (
Figure 1 ). Gray matter volume reduction in these three regions was related to the severity of psychopathology (
Table 2 ).
Gray matter deficits in schizophrenia patients were reported in approximately 50 brain regions in one meta-analysis
(1) . Among those regions, volume reduction in the superior temporal gyrus had 100% replicability with region-of-interest measurements
(35) and approximately 50% replicability with voxel-based morphometry approaches in previous studies
(1) . However, decreased gray matter volume in the right anterior cingulate gyrus and right middle temporal gyrus had replicability with voxel-based morphometry approaches of approximately 24% and 7%, respectively, in previous studies
(1) . The inhomogeneous findings of different voxel-based morphometry studies may be related to numerous confounds, including illness chronicity and antipsychotic medication effects
(4,
5,
36,
37) as well as methodological differences (e.g., smoothing kernel)
(1) .
The study of untreated first-episode schizophrenia is especially useful for understanding the disorder-related clinical and neurophysiological correlates of morphometric changes. To our knowledge, only three voxel-based morphometry studies
(3,
6,
38) have, to date, recruited antipsychotic-naive schizophrenia patients, all with a smaller sample size than that of the present study. Each of these previous studies reported decreased gray matter in the cingulate gyrus, and none found alterations in the right middle temporal gyrus. However, findings for the superior temporal gyrus differed among the three studies. To our knowledge, the present study is the first to demonstrate that gray matter deficits in the right middle temporal gyrus and right superior temporal gyrus may be present during a very early stage of schizophrenia.
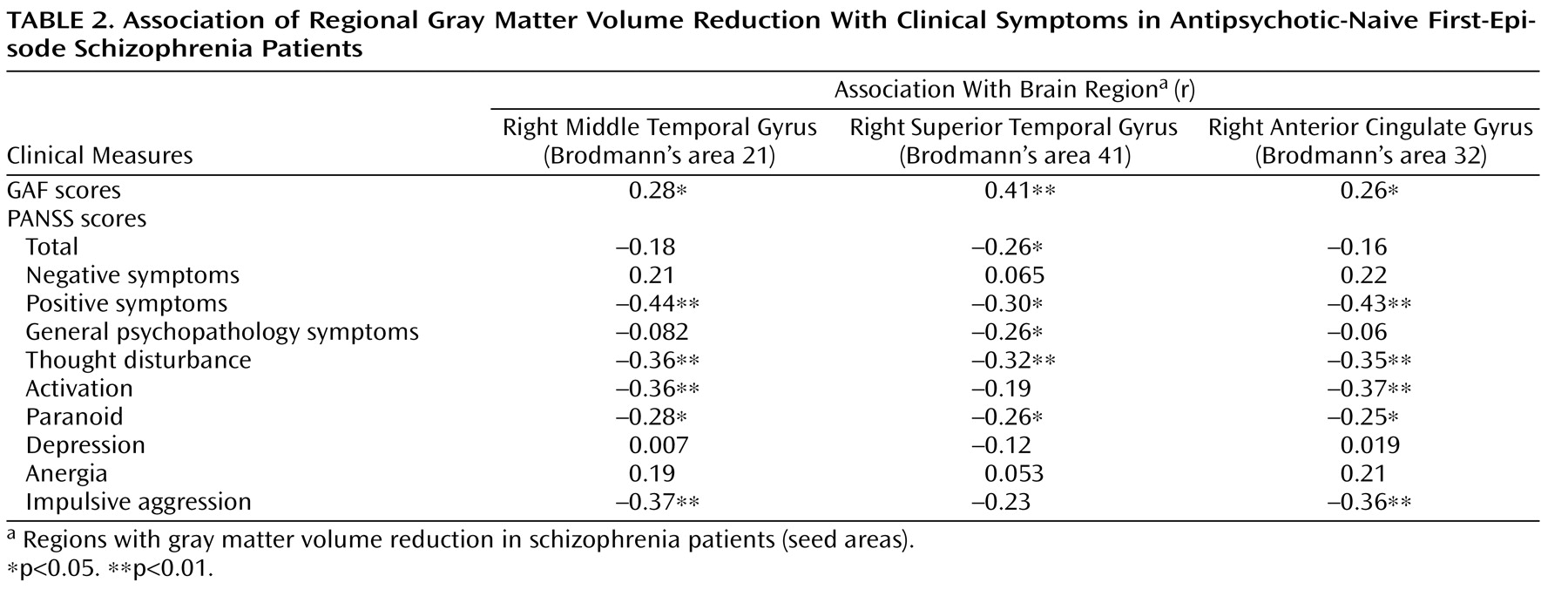
In the present study, we also found positive correlations between gray matter volume values in the three brain regions with anatomic deficits (right anterior cingulate gyrus, right middle temporal gyrus, and right superior temporal gyrus) and GAF scores. This finding confirms that the gray matter abnormalities were associated with increased levels of psychopathology and functional impairment among antipsychotic-naive first-episode schizophrenia patients
(39) . In a previous study using single photon emission computed tomography, activity in the anterior cingulate gyrus was reported to be correlated with some positive symptoms, such as delusion, in antipsychotic-naive schizophrenia patients
(40) . In the present study, we utilized MRI to show that gray matter volume in the anterior cingulate gyrus was correlated with the psychopathology of schizophrenia, since there were negative correlations of gray matter volume in the right anterior cingulate gyrus with PANSS scores for positive symptoms, thought disturbance, activation, paranoia, and impulsive aggression.
The role of volume reduction in the superior temporal gyrus as it relates to negative symptoms
(8) and thought disorder
(2,
41) in schizophrenia has been discussed in previous studies, although some nonreplication has also been reported
(9,
10) . Studies of patients with familial and nonfamilial schizophrenia have suggested that superior temporal gray matter volume reduction may be a familial phenotype
(42) and may have a neurodevelopmental origin, since decreases in superior temporal gray matter volume in these studies occurred among unaffected individuals for whom illness progression effects would not have been a potential cause of morphometric abnormality
(42,
43) . Although decreased gray matter volume in the middle temporal gyrus has been rarely reported in the literature
(1), recent structural
(44) and functional
(41) studies have shown correlations between the severity of symptoms and severity of abnormalities in the middle temporal gyrus among first-episode schizophrenia patients. In the present study, our results confirm the presence of neuroanatomic abnormalities in the temporal and cingulate cortex and further confirm the role of these areas in the pathology of schizophrenia by demonstrating correlations of gray matter volume reduction with symptom profiles (
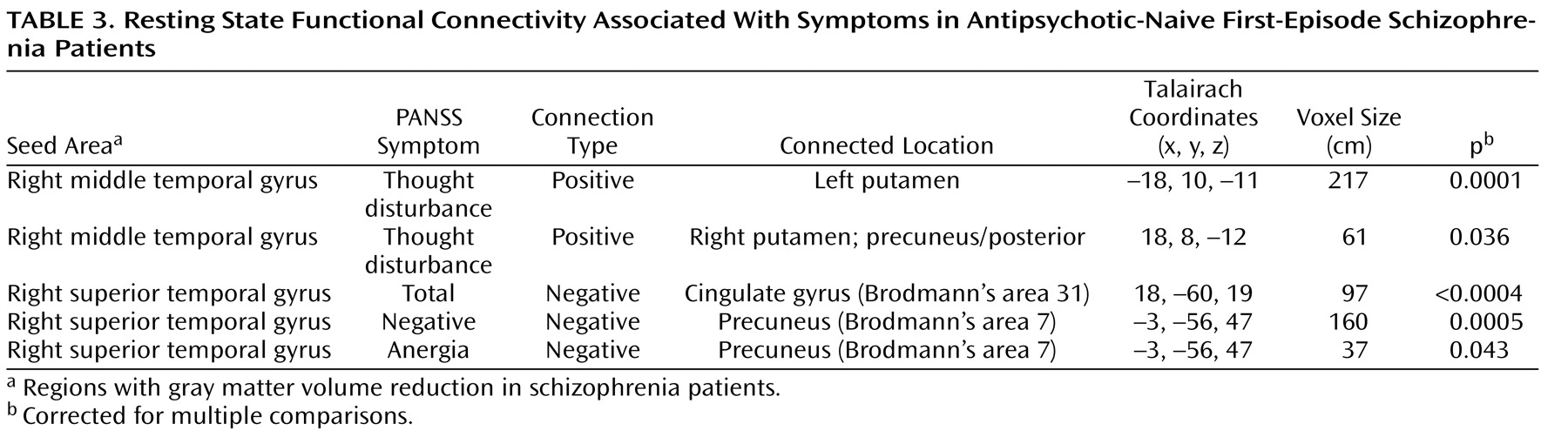
Table 3 ).
Alterations of functional connectivity in schizophrenia has been treated as a potential systems-level substrate of the disorder. Through the use of resting-state fMRI, previous studies
(22,
23,
45) have reported alterations of functional connectivity in schizophrenia patients. Decreased fronto-parietal, fronto-cingulate, and fronto-thalamic connectivity have also been reported
(22,
23,
45) . However, the findings have not been consistent. These previous studies utilized different a priori anatomic regions as seeds based on the specific hypotheses of the investigations. Furthermore, since regional changes in resting state brain physiology are known to occur after antipsychotic treatment
(46,
47), previous functional connectivity studies, including those of first-episode schizophrenia patients who received treatment
(22,
23,
45), may have detected effects of antipsychotic medication rather than direct illness effects on brain function. Longitudinal studies of patients evaluated before and after treatment are needed in order to assess whether effects of antipsychotic medication or direct illness effects on brain function are present.
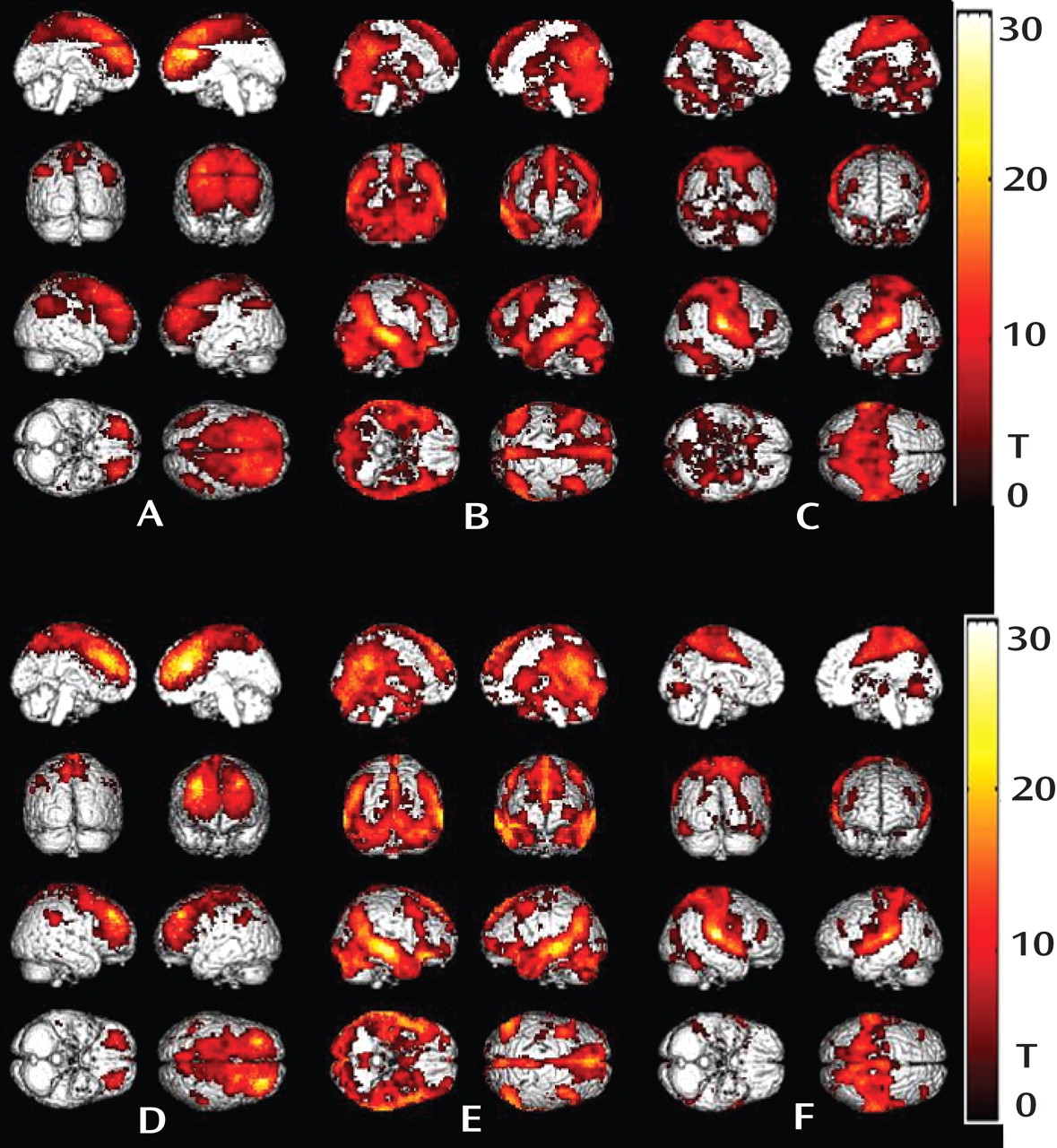
In the present study, regions with abnormal gray matter volume were used as seeds for functional connectivity analysis. Surprisingly, patterns of resting state functional connectivity for all three structurally abnormal regions (right anterior cingulate gyrus, right middle temporal gyrus, and right superior temporal gyrus) were similar among antipsychotic-naive first-episode schizophrenia patients and healthy comparison subjects (
Figure 2 ). In addition, we did not observe altered functional connectivity with the dorsal lateral prefrontal cortex in our antipsychotic-naive schizophrenia sample, which has been found in patients who received treatment in previous studies
(22,
34) . Thus, gray matter volume reduction in the three regions—although related to symptoms—did not appear to significantly alter the integration of affected brain regions within their corresponding functional networks, at least with regard to resting state indices of functional connectivity. However, correlations were found between the clinical symptoms and functional connectivity of the middle temporal gyrus with the putamen and between the clinical symptoms and functional connectivity of the superior temporal gyrus with the precuneus. Middle temporal connectivity with the putamen among first-episode schizophrenia patients was associated with thought disturbance, while superior temporal gyrus connectivity with the precuneus was associated with PANSS total scores as well as scores for negative symptoms and anergia (
Table 3 ). This suggests that heightened patterns of common activity between the basal ganglia and middle temporal gyrus may be associated with increased levels of thought disorder, an association that is perhaps mediated by increased striatal metabolic or dopaminergic activation that can occur during acute episodes of illness
(48) . In contrast, functional integration of the superior temporal gyrus with the precuneus was associated with negative symptoms, which is an association that might be related to reduced attention or impaired processing of real-life events
(49) .
To our knowledge, the present study is the first to identify two different patterns of associations between brain abnormalities and symptoms of first-episode schizophrenia. First, volume reduction in the right anterior cingulate gyrus, right middle temporal gyrus, and right superior temporal gyrus was directly related to the severity of several dimensions of psychopathology. Second, functional connectivity levels of the right middle temporal gyrus and right superior temporal gyrus were associated with symptom severity and thus may have clinical significance in some more severely ill patients. However, since schizophrenia patients were not followed over time, we were unable to determine whether the reported relations between symptom severity and neurophysiological and morphometric measures were restricted to periods of acute psychosis or were patterns that persisted during recovery.
The two patterns of associations between anatomic deficits and symptom severity among first-episode schizophrenia patients suggest that there may be important clinical and, perhaps, neuropsychological effects associated with disorder-related morphometric changes in the right middle temporal gyrus and superior temporal gyrus that could be examined in future studies of schizophrenia. For example, a recent study by Weinstein et al.
(41) revealed that the association between decreased gray matter volume in the left superior temporal gyrus and the severity of thought disorder was mediated by the level of activation in the superior and middle temporal gyri during language processing.
Several methodological limitations regarding the use of voxel-based morphometry should be considered when interpreting the results of the present study. First, although optimized voxel-based morphometry was employed, which minimizes the contamination of brain tissue with nonbrain voxels relative to standard voxel-based morphometry
(33), findings from our sample of Chinese patients may have affected the accuracy of normalization because the generic MNI template differs structurally in the brains of non-Caucasian populations
(50) . However, since both schizophrenia patients and healthy comparison subjects were of a similar ethnic background, it is unlikely that the group differences observed were affected by ethnic factors. Second, some of the differences in gray matter volume may have resulted from systematic morphological differences between the two groups, since specific patterns of abnormal morphology may result in group-specific misregistration. In such a case, there can be increased sensitivity to systematic shape differences attributable to misregistration from the spatial normalization step
(51) . Systematic differences in morphology are one of several potential differences between schizophrenia patients and healthy comparison subjects that may be detected by voxel-based morphometry, and they reflect a real difference between data obtained from different populations but may not necessarily be the result of changes in gray matter volume
(52) . In addition, compared with the classic region of interest method, previous studies
(53,
54) of individuals with schizophrenia have indicated that the results of voxel-based morphometry and region of interest are generally consistent, and voxel-based morphometry has proven to be somewhat more sensitive than region of interest methods. Last, we also recognize that voxel-based morphometry may not be sensitive to a disease that likely affects brain structure in nonlinear ways, as first demonstrated by Davatzikos
(55) . Although voxel-based morphometry methods used to accommodate nonlinearities have been proposed
(56), it is important to acknowledge that voxel-based morphometry is not a surrogate for multivariate volumetric analyses that have been specifically developed to characterize highly distributed nonlinear changes.
In conclusion, the present study applied morphometry analysis and resting-state functional connectivity to examine their relationship with the clinical features of first-episode schizophrenia. Our findings document that symptoms in antipsychotic-naive patients with first-episode schizophrenia are associated with both structural deficits and alterations in the functional brain networks of regions with gray matter volume reduction. However, regions with gray matter volume reduction did not have significantly altered resting state functional connectivity with other brain regions. Our results provide support for future efforts to combine anatomical and functional data to explore the complex disorder of schizophrenia.