For several decades the fields of psychiatry and psychopharmacology have been engaged in a desperate search for new drugs with novel mechanisms of action to improve the treatment of schizophrenia. From reviewing our pharmacopeia for schizophrenia it appears that this has been a daunting, if not quixotic, quest, particularly if we add the challenge of developing drugs that act on targets other than dopamine. This could likely be due to the fact that most of the discoveries that have historically propelled our field forward in therapeutics have come through serendipity and not from a process of rational drug development in which drugs are developed on the basis of targets derived from theories of etiology or pathophysiology. In this issue of the
Journal, the results of studies by Freedman et al.
(1) and Shekhar et al.
(2) with two novel compounds acting through different mechanisms as cholinergic agonists suggest that the tide may be turning and that rational drug development may have arrived in psychiatry.
It has long been known that acetylcholine plays an important role in cognition and that impaired cholinergic transmission contributes to the cognitive deficits in Alzheimer’s disease
(3) . There is evidence for decreased numbers of both muscarinic and nicotinic receptors in schizophrenia
(4,
5), and a functional polymorphism of the α
7 nicotinic receptor has been linked genetically to this disorder
(6) . Moreover, acetylcholine modulates dopamine transmission in the striatum
(7,
8), where dopamine dysregulation may contribute to both positive and negative symptoms
(9), and in the cortex
(10), where dopamine transmission deficits have been postulated to contribute to cognitive deficits
(9,
10) . These findings, along with the heavy consumption of nicotine by patients with schizophrenia
(11) and the suggestion that muscarinic receptor agonism by clozapine or its active metabolite may underlie its potential amelioration of negative and cognitive symptoms, have raised interest in cholinergic agonists to treat schizophrenia and its cognitive deficits
(10,
12) .
Cholinergic transmission is mediated by two families of receptors: 1) nicotinic acetylcholine receptors, which are ligand-gated ion channels formed by pentameric combinations of different α and β subunits, as well as homomeric α
7 nicotinic receptors, and 2) the muscarinic receptors M
1 –M
5 (8,
13) . Activation of nicotinic receptors leads to a rapid increase in sodium and/or calcium conductance that increases neuron activity and neurotransmitter release
(8) . Muscarinic receptor signaling, on the other hand, is mediated by G proteins exerting slower but potentially more sustained effects
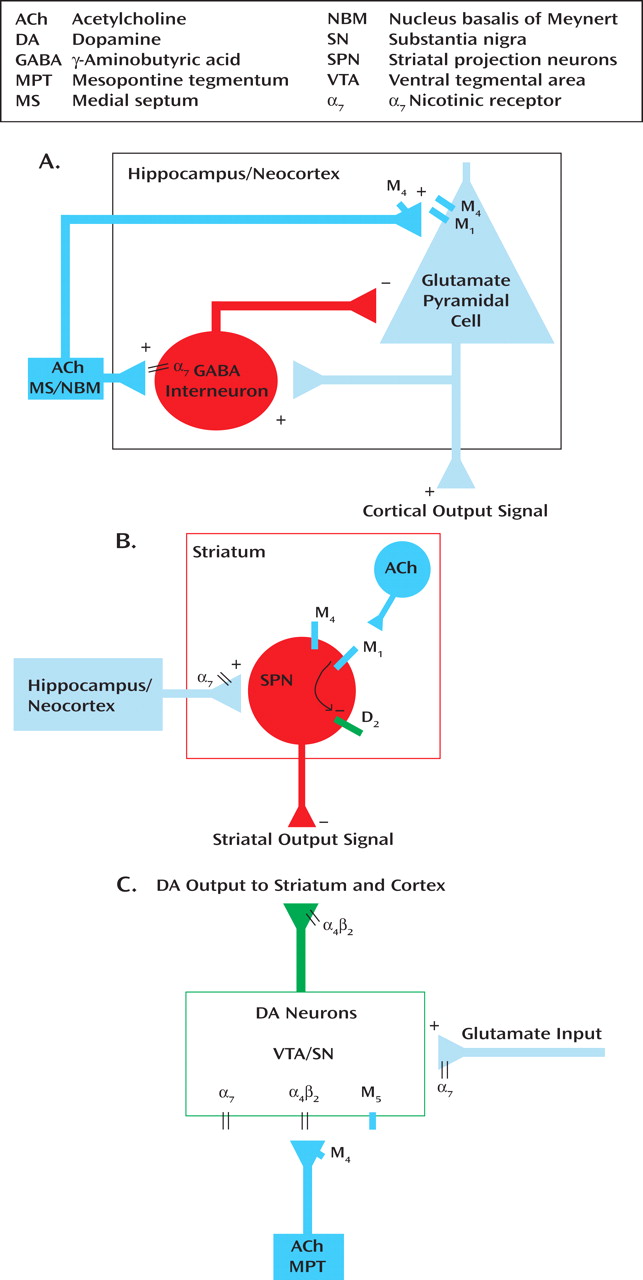
(13) . Acetylcholine is widely distributed in the brain; however, its actions in the neocortex, hippocampus, and striatum, and at midbrain dopamine neurons, are of perhaps the greatest relevance to cholinergic therapies in schizophrenia. The neocortex and hippocampus receive cholinergic input from basal forebrain neurons located primarily in the nucleus basalis of Meynert and medial septum, respectively
(3) (
Figure 1 A). The striatum, which receives massive cortical input, is highly concentrated in acetylcholine that is released from local cholinergic interneurons
(8) (
Figure 1 B). The neocortex, hippocampus, and striatum receive dopamine input from midbrain dopamine cell groups, including the ventral tegmental area and substantia nigra
(9) (
Figure 1 C). The activity of these dopamine neurons is potently regulated by a cholinergic/glutamatergic input from the mesopontine tegmentum
(7,
8) (
Figure 1 C).
Numerous challenges have limited the development of drugs targeting CNS cholinergic receptors. Because of the similarity of their binding sites, it has been difficult to design drugs with high specificity for the various subtypes of muscarinic and nicotinic receptors. Xanomeline, for example, is an M
1 and M
4 agonist but is also a high-affinity M
5 antagonist
(13), as well as a potent agonist of 5-HT
1A and 5-HT
1B receptors and an antagonist of 5-HT
2 receptors
(14) . Selective targeting of muscarinic CNS receptors over peripheral receptors has also been a significant problem, since the tolerability of drugs is often limited by peripheral side effects, such as those affecting cardiac or gastrointestinal function. Targeting nicotinic receptors is complicated not only by the limited availability of compounds selective for particular subtype combinations, but also by the development of rapid agonist-induced receptor desensitization, which makes it difficult to treat over the long term with an agonist and maintain a physiological effect. This latter property would entail the need for any therapeutic agent acting through the nicotinic system to be very frequently administered (hence the chain smoking often seen in schizophrenia patients) unless a compound can in effect “thread the pharmacodynamic needle” and stimulate the receptor without causing it to become desensitized.
Despite these formidable challenges, cholinergic agonists remain attractive targets for drug innovation. For these reasons, the reports of studies with cholinergic agonist drugs are most welcome and of great significance. The most critical stage in the development of any new compound based on a theory (as opposed to a new compound that copies an existing class of drugs that is already known to work) is the “proof of concept” study in which the theory-based experimental compound (supported by preclinical studies) is put to the test of whether it will work in patients. In the reports by Freedman et al.
(1) and Shekhar et al.
(2) we have the glimmerings of positive results for the cholinergic strategy.
Freedman et al.
(1) tested a cholinergic nicotinic partial agonist selective for the a
7 receptor (DMXB-A) in 31 clinically stable outpatients with chronic schizophrenia receiving maintenance antipsychotic medication, using a placebo-controlled crossover design. After a lead-in week of placebo to assure compliance, patients were randomly assigned to receive either placebo or one of two doses (75 mg b.i.d. or 150 mg b.i.d. p.o.) of DMXB-A for 4 weeks. Patients were evaluated at baseline with the MATRICS Consensus Cognitive Battery, which was especially developed by an NIMH-sponsored project to assess the effects of treatment on cognitive function in schizophrenia patients, and with two measures of psychopathology, i.e., the Brief Psychiatric Rating Scale (BPRS) and Scale for the Assessment of Negative Symptoms (SANS). Following this initial trial, patients underwent a 1-week washout period and then received another of the three treatment options for 4 weeks, followed by another 1-week washout and then 4 weeks of the last of the three treatment options. Patients were evaluated for cognitive function and psychopathology at the beginning and end of each 4-week treatment period. Side effects were also assessed.
The investigators did not find significant improvement on their primary outcome measure of cognition, the MATRICS battery, but they did see a therapeutic effect on negative symptoms measured by the SANS. Moreover, data analysis revealed improvement in the MATRICS scores in all treatment groups (including placebo) over the course of the study, leading them to conclude that there may have been a significant practice effect (given that the patients took the test four times over the 16-week period). Investigators thought this practice effect might have obscured the potential for treatment effects to be seen on this measure. For this reason they examined the MATRICS scores in just the first 4-week trial of the study and found significant improvement in attention/vigilance with both doses of DMXB-A, significant improvement in working memory at the 75-mg b.i.d. dose, and trend-level improvement in working memory with the 150-mg b.i.d. dose. Although these results fall short of the original hypothesis of the study, they are encouraging, particularly given the limitations of the DMXB-A compound that was used to test the nicotinic strategy.
In a separate proof-of-concept study of xanomeline, a muscarinic cholinergic agonist with relative selectivity for M
1 and M
4 receptors, Shekhar et al.
(2) randomly assigned 20 inpatients with schizophrenia and active psychotic symptoms to placebo or xanomeline monotherapy in an escalating dose to 75 mg t.i.d. for 4 weeks. The primary outcome measure was behavioral improvement measured by the Clinical Global Impression (CGI), but psychopathology and cognitive function were assessed as well. Patients who received xanomeline improved significantly more than those taking placebo on the CGI, BPRS, and Positive and Negative Syndrome Scale (PANSS), and scores on the PANSS positive and negative subscales improved at trend levels. In addition, patients showed greater improvement with xanomeline on the cognitive battery.
These results, though preliminary, encourage our interest in the cholinergic strategy to develop therapeutics for schizophrenia. Although DMXB-A is unlikely to ever be commercialized and may not even be the optimal compound to test the nicotinic α
7 hypothesis, in this study it was able to demonstrate the value of this target and the utility of this approach. Moreover, it represents an impressive example of rational drug development in that it derives from an etiologic hypothesis conceived and promulgated by the Freedman group
(6) . Indeed, the development of DMXB-A was an outgrowth of the elegant series of studies on the role of the nicotinic system in the etiopathogenesis of schizophrenia.
The development of xanomeline represents an effort to address the cholinergic deficit in Alzheimer’s disease
(15,
16) . The results of a previously completed proof-of-concept study in Alzheimer’s disease were positive in terms of cognition and psychosis, but poor tolerability in the form of gastrointestinal side effects was a limiting factor
(16) . Both xanomeline and DMXB-A produced side effects in the current studies, including in the gastrointestinal system, but these occurred at tolerable levels and led to no more dropouts than occurred in the placebo groups.
Preclinical studies suggest that the nicotinic α
7 agonists, such as DMXB-A, induce their effects on cognition through activation of hippocampal interneurons
(17) (
Figure 1 A). In addition to their potential cognitive benefits from this and other effects in the neocortex and hippocampus, nicotinic agonists also alter dopaminergic transmission. Acetylcholine is a critical regulator of dopamine neuron firing, through its action at postsynaptic α
4 β
2 and α
7 nicotinic receptors, as well as through α
7 receptors on the terminals of glutamate terminals that excite the dopamine neurons
(7,
8) (
Figure 1 C).
Further evidence suggests that the beneficial effects of muscarinic cholinergic agonists in schizophrenia may result from their impact on the profound interactions between dopaminergic and cholinergic signaling at the level of both the cell body and terminal. The cognitive benefits of xanomeline are thought to result from its stimulation of M
1 receptors in the neocortex and hippocampus (
Figure 1 A), an action that facilitates acetylcholine and dopamine release in these regions
(10) (
Figure 1 C). However, the antipsychotic effects may be mediated through M
1 /M
4 stimulation of striatal neurons and antagonism of M
5 receptors in the ventral tegmental area. In the striatum, stimulation of M
1 receptors opposes the action of dopamine D
2 receptors, rendering D
2 -expressing striatal projection neurons more responsive to cortical inputs
(18) (
Figure 1 B). Xanomeline, perhaps through M
4 receptors, also counteracts the effects of dopamine receptor stimulation in behavioral tasks
(13) . In the ventral tegmental area, xanomeline may antagonize M
5 receptors, preventing prolonged dopamine release in the striatum following activation of these dopamine neurons
(19) (
Figure 1 C). Since xanomeline also acts at multiple other receptors, more selective compounds will be necessary to parse out the effects of the individual receptors.
These studies provide an important signal of the potential of cholinergic agonists as novel therapeutic agents in schizophrenia. Moreover, they follow the recent publication of positive findings on the use of a metabotropic glutamate receptor 2/3 agonist monotherapy, a treatment based on the glutamate hypothesis of schizophrenia
(20) . This augurs well for the field. Only further pursuit of these targets and replication of these results will confirm the validity of these novel strategies. Nevertheless, the encouraging results with these cholinergic agonists suggest that the tide may be turning in experimental therapeutics for schizophrenia and that rational drug development for the field of psychiatry may have finally arrived.