Generalized anxiety disorder is characterized by frequent and difficult-to-control episodes of free-floating anxiety or worry (
1). Cognitive models suggest that worry reflects an overlearned compensatory strategy for dulling emotional experience (
2). However, it is unclear why emotional experiences in patients with generalized anxiety disorder necessitate the use of this cognitively costly regulatory strategy. Seen from the perspective of emotion regulation, patients with generalized anxiety disorder may resort to worry because of an underlying abnormality in regulating emotional processing (
3–
5).
Studies of other anxiety disorders predict that the amygdala in patients with generalized anxiety disorder would be hyperreactive to negative emotional stimuli (
6). Two studies of adolescents with generalized anxiety disorder support this prediction (
7,
8), and two similar studies of adults do not (
9,
10). One study of adults with generalized anxiety disorder even found amygdalar hyporesponsiveness to faces with fearful expressions (
9), although another study found nonspecifically exaggerated amygdalar reactivity to warning cues preceding either aversive or neutral pictures (
11). We recently reported on an intra-amygdalar perturbation at a subregional level in adults with generalized anxiety disorder (
12).
Studies of generalized anxiety disorder also point to an important role for the prefrontal cortex. Paulesu et al. (
13) found that patients were unable to normalize activation in the dorsal anterior cingulate cortex and dorsomedial prefrontal cortex after a worry induction. Pediatric studies have noted exaggerated ventrolateral prefrontal activation to emotional stimuli in patients (
7,
14), with the degree of hyperactivation negatively correlated with anxiety scores (
14), which suggests a compensatory role for the hyperactivation. We also found evidence for compensatory coupling of a lateral prefrontal cortical executive network with the amygdala in patients (
12). Thus, while abnormalities appear to exist within both limbic and prefrontal regions in generalized anxiety disorder, the nature of these abnormalities remains poorly understood.
Use of an experimental paradigm in which emotion regulation can be tracked from trial to trial may be a fruitful approach to understanding generalized anxiety disorder. We recently reported (
15,
16) on a facial affect identification emotional conflict task in which healthy volunteers were asked to identify the expression of a face (fearful or happy) while ignoring an overlying emotion word ("fear" or "happy") that either matched (congruent) or conflicted (incongruent) with the facial expression. Reaction time interference by emotionally incongruent stimuli was seen in nearly every participant (
15,
16). Interestingly, there is less conflict, indexed by faster reaction times, for incongruent trials if they are preceded by an incongruent trial than if they are preceded by a congruent trial (
15–
20), which suggests that the emotional conflict generated by incongruency on the previous trial activates a regulatory mechanism that leads to improved emotional conflict regulation on the current incongruent trial (
16,
21–
23), thus optimizing task performance. We termed this across-trial effect "emotional conflict adaptation" (
15,
16), in reference to the label previously applied to similar congruency sequence effects observed in nonemotional conflict tasks (
21). Likewise, performance on postcongruent congruent trials is often superior to that on postincongruent congruent trials (
21).
To date, the cognitive model that best accounts for the conflict adaptation effect, after eliminating potential confounders (
24), is the "conflict monitoring hypothesis" (
17–
23,
25,
26). According to this model, conflict is continuously evaluated, such that greater conflict regulation can be flexibly recruited as required by the amount of conflict. Thus, the conflict-monitoring hypothesis distinguishes between two important functions—conflict evaluation and conflict regulation. Many studies have examined the regions associated with these functions during adaptation to nonemotional conflict (e.g., color-word Stroop or flanker tasks) by comparing activity during incongruent trials that differ only with respect to whether they were preceded by a congruent or an incongruent trial (
17,
18,
20–
23,
25,
26). Regions whose activity tracks the amount of conflict (i.e., postcongruent incongruent trials > postincongruent incongruent trials) have been interpreted as conflict evaluation regions (
17,
18,
20–
23,
25,
26). Regions showing the opposite effect (postincongruent incongruent trials > postcongruent incongruent trials) have been interpreted as conflict regulation regions (
17,
18,
20–
23,
25,
26), as activity in these regions is greatest when conflict is minimized through regulation. Because these contrasts compare physically identical incongruent trials, the behavioral and neural effects differ only by virtue of expectation created by conflict on the previous trial (
17–
23,
25,
26).
In previous studies (
15,
16) we applied this logic to the analysis of adaptation in a novel emotional conflict task. Greater activity during postincongruent incongruent trials (i.e., regulation-related) was seen in the pregenual anterior cingulate, and this was accompanied by strong negative coupling between the pregenual cingulate and the amygdala. These findings are consistent with other contexts in which emotion regulation is observed (
27–
29). By contrast, greater activity during postcongruent incongruent trials (i.e., evaluation-related) was seen in the amygdala and the dorsal anterior cingulate/dorsomedial prefrontal cortex.
We also compared activations during emotional conflict adaptation with those during nonemotional conflict adaptation (gender identification with the same emotional faces, while ignoring gender words overlaid on the faces) to determine the specificity of activations for emotion. Pregenual cingulate activation and coupling with the amygdala was specific to emotional conflict adaptation, whereas dorsal anterior cingulate/dorsomedial prefrontal cortex activation was shared by emotional and nonemotional conflict (
15,
16), consistent with the role of these latter regions in the evaluation of conflict in many other studies of nonemotional conflict adaptation (
17,
20,
25,
26).
Considering that the clinical phenomenology of generalized anxiety disorder suggests that a deficit in the regulation of emotional processing is at the core of this disorder, we hypothesized that patients would show abnormalities in adapting to emotional conflict in our task. Additionally, to better understand emotional conflict adaptation more generally, and thus enhance interpretation of abnormalities in patients, we investigated in a separate cohort of healthy volunteers whether they were aware of these trial-to-trial adaptation effects and thus whether conscious attention is required for this process. We hypothesized that participants would not be aware of the adaptation effect and thus that this process is carried out at an implicit level.
Method
Participants
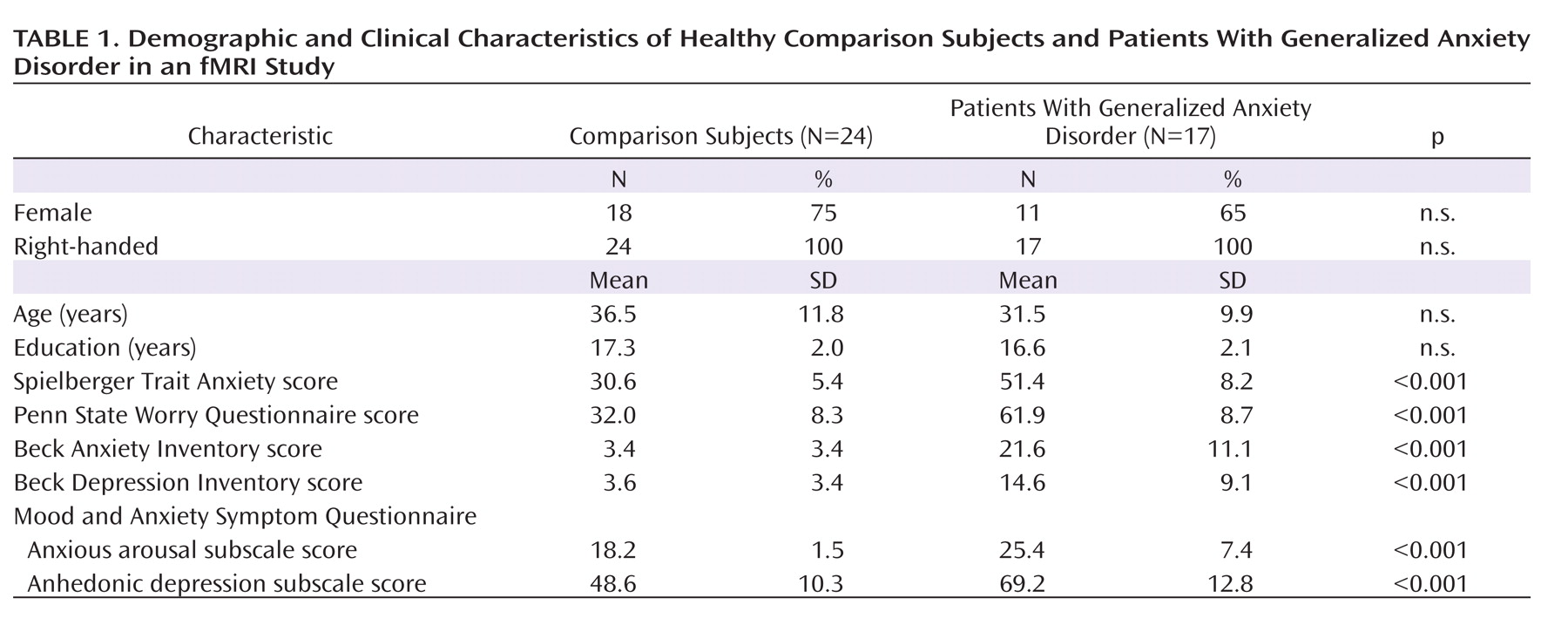
A total of 41 individuals, recruited locally through online advertisements, participated in the functional MRI (fMRI) component of this study; all provided informed consent. DSM-IV-based psychiatric diagnoses were determined through both an informal clinical interview with a psychiatrist and the Mini-International Neuropsychiatric Interview, a structured diagnostic interview (
30,
31). Generalized anxiety disorder was the primary diagnosis for all patients, in terms of both onset and severity. Exclusion criteria were major depressive disorder, bipolar disorders, psychotic disorders, substance abuse, and posttraumatic stress disorder; a history of a neurological disorder, head trauma, or loss of consciousness; claustrophobia; or regular use of benzodiazepines, opioids, or thyroid medications. No patient was taking regular psychiatric medications or had used a benzodiazepine within 48 hours of the scan. No patient had ever received an evidence-based structured psychotherapy, and only five patients had ever received antidepressant medication. Nine patients had no comorbid disorders, five had one comorbid disorder (two with dysthymia and three with social anxiety), three had two comorbid disorders (two with social anxiety and panic disorder, and one with social anxiety and obsessive-compulsive disorder), and none had more than two comorbid disorders. All comparison subjects were free of any current or past axis I conditions or psychiatric medications. All participants completed the Spielberger State-Trait Anxiety Inventory (
32), the Penn State Worry Questionnaire (
33), the Beck Anxiety Inventory (
34), the Beck Depression Inventory (
35), and the Mood and Anxiety Symptoms Questionnaire (
36,
37), from which the anxious arousal and anhedonic depression subscales were used. Resting state data from nine of the healthy comparison subjects and 10 of the patients were included in a previous study (
12). The behavior-only study was conducted on a group of 19 healthy volunteers (mean age=25.2 years [SD=1.0]; 13 of them women) that did not overlap with the healthy comparison group involved in the fMRI study.
Experimental Paradigm
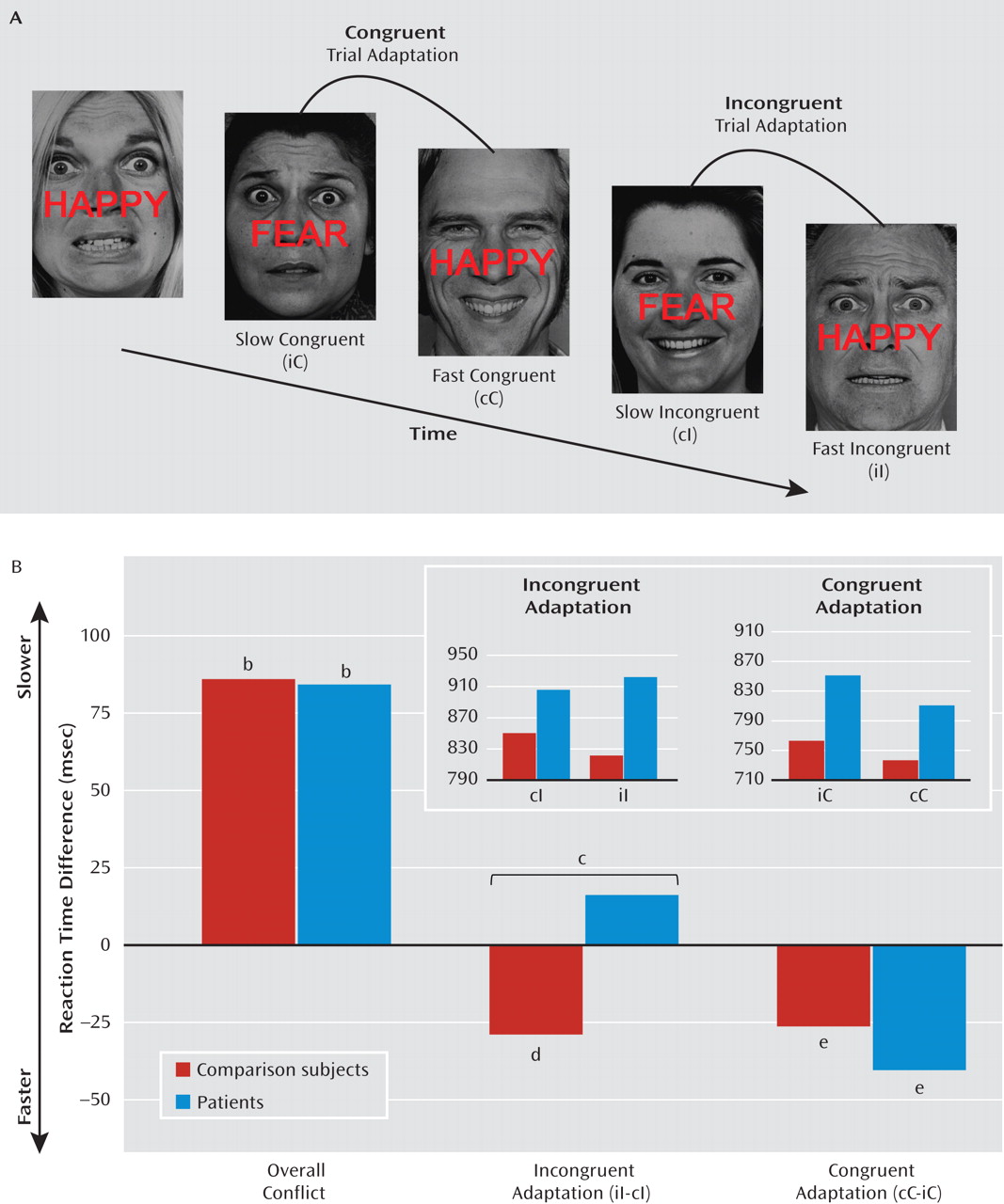
The emotional conflict task was performed as previously described (
15,
16). Stimuli were presented with the Presentation software package (Neurobehavioral Systems, http://nbs.neuro-bs.com) during fMRI scanning and displayed through a custom-built MRI-compatible projection system. The task consisted of 148 presentations of happy or fearful facial expression photographs drawn from the set of Ekman and Friesen (
38), overlaid with the words "FEAR" or "HAPPY." Stimuli were presented for 1,000 msec, with a varying interstimulus interval of 3000–5000 msec (mean=4,000 msec), in a pseudorandom order, counterbalanced across trial types for expression, word, response button, and gender. Participants indicated facial affect with a button press response. Behavioral data were analyzed in SPSS (SPSS, Inc., Chicago). For the behavior-only task, a questionnaire was administered after the task to assess participants' awareness of the conflict adaptation effect.
fMRI Data Acquisition
Images were acquired on a 3-T GE Signa scanner using a custom-built head coil. Twenty-nine axial slices (4.0 mm thickness with a 0.5 mm gap) were acquired across the whole brain using a T
2*-weighted gradient echo spiral pulse sequence (repetition time=2,000 msec, echo time=30 msec, flip angle=80°, interleave=1, field of view=22 cm, matrix=64×64) (
39). To reduce blurring and signal loss arising from field inhomogeneities, an automated high-order shimming method based on spiral acquisitions was used before acquisition of functional MRI scans (
40). A high-resolution T1-weighted three-dimensional inversion recovery spoiled gradient-recalled acquisition in the steady state MRI sequence was used with the following parameters: inversion time=300 msec, repetition time=8 msec; echo time=3.6 msec; flip angle=15°; field of view=22 cm; 124 slices in coronal plane; matrix=256×192; number of excitations=2; acquired resolution=1.5×0.9×1.1 mm. The images were reconstructed as a 124×256×256 matrix.
fMRI Data Analysis
The first five volumes were not analyzed to allow for signal equilibration effects. A linear shim correction was applied separately for each slice during reconstruction using a magnetic field map acquired automatically by the pulse sequence at the beginning of the scan (
39). Functional MRI data were then preprocessed using the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (MathWorks, Inc., Natick, Mass.). Images were realigned to correct for motion, slice timing-corrected, spatially transformed to the Montreal Neurologic Institute coordinate system (
41), resampled every 2 mm, and smoothed with a 6 mm full-width at half-maximum Gaussian kernel. During preprocessing, the effects of global signal were also removed separately for each voxel (
42). A 128-second temporal high-pass filter was applied to the data, and temporal autocorrelation was estimated using a first-order autoregressive model. Separate regressors for the stimulus events (convolved with a canonical hemodynamic response function) were created for postcongruent incongruent trials, postincongruent incongruent trials, postcongruent congruent trials, and postincongruent congruent trials, with error and posterror trials modeled separately. Additional regressors of no interest corresponding to the six motion parameters were also included. This model was applied to normalized data in the context of a generalized linear model (
43) and submitted to group-level random-effects analyses using two-sample t tests. As described previously and above (
15,
16), our contrasts took advantage of the conflict adaptation effect to compare activity during incongruent (or congruent) trials for which behavior differs by virtue only of expectation created by the previous trial type (e.g., postincongruent incongruent trials minus postcongruent incongruent trials).
For the psychophysiologic interaction analyses (
44), we extracted for each participant a deconvolved time course from the healthy comparison group-level contrast of postincongruent incongruent trials minus postcongruent incongruent trials (p=0.01). Activity within the amygdala was then regressed on a voxel-wise basis against the product of this time course and the vector of the psychological variable of interest, with the physiological and the psychological variables serving as regressors of no interest, along with the six motion parameters. The results were then taken to a random-effects group analysis using two-sample t tests.
We report results within independently defined a priori regions of interest based only on our prior data with the emotional conflict task (
15,
16) using small-volume corrections (
45) (p<0.05, family-wise error-corrected). Specifically, to determine the optimal center coordinates for spherical regions of interest, we averaged the medial prefrontal or anterior cingulate peak coordinates from our previous studies of healthy volunteers scanned with the identical task and created spheres (intersected with a dilated gray matter mask) of 12 mm radius around these coordinates for the dorsomedial prefrontal cortex (x=5, y=33, z=31; 6,848 mm
3) and the pregenual cingulate (x=–10, y=42, z=0; 5,696 mm
3). In this way, our statistical inferences in this study are directly driven by a priori hypotheses about spatial location of effects of interest from our previous studies. The amygdala region of interest corresponded to the left and right amygdala in the Wake Forest University PickAtlas (left: 12×10×18 mm, 1,264 mm
3; right: 14×12×16 mm, 1,288 mm
3) (
46). Results are displayed within these regions of interest only.
Results
Behavior
Our patient and comparison groups were well matched for age, gender, handedness, and education (
Table 1). No group difference was observed in either overall reaction times or accuracy (comparison group: reaction time=793 msec [SD=22], accuracy=94.9% [SD=0.8]; patient group: reaction time=872 msec [SD=58], accuracy=93.4% [SD=1.4]). Emotional conflict slowed reaction times similarly in both groups (incongruent minus congruent trial difference), including in all healthy comparison subjects and in all but one patient (comparison group: t=6.77, df=23, p<0.000001; Cohen's d=1.4; patient group: t=5.82, df=16, p<0.00005; d=1.4); group comparison: t=0.09, df=39, p>0.9; see
Figure 1B). There was a significant group difference in across-trial reaction time adjustment related to emotional conflict adaptation during incongruent trials (t=2.39, df=39, p<0.05; d=0.8; see Figure 1B). This effect was driven by the predicted faster performance of healthy comparison subjects on postincongruent incongruent trials than on postcongruent incongruent trials (t=2.19, df=23, p<0.05; d=0.45, see Figure 1B). Patients with generalized anxiety disorder failed to show this effect. By contrast, for congruent trials, exposure to an immediately preceding congruent trial produced similarly significant reaction time facilitation in both groups (comparison group: t=3.26, df=23, p<0.005; d=0.66; patient group: t=2.87, df=16, p=0.01; d=0.7; group comparison: t=0.93, df=39, p>0.35; see Figure 1B; see also Table S1 in the data supplement that accompanies the online edition of this article).
Finally, we asked a separate group of healthy volunteers whether they were aware of any pattern across trials that might help or hinder their performance. No participant mentioned previous trial conflict. In addition, discrimination in a forced-choice question of whether performance on a current incongruent trial was improved by a previous incongruent trial compared to a previous congruent trial did not differ from chance (p>0.25), which suggests that conscious awareness of the adaptation phenomenon is not required for successful adaptation.
Abnormal Medial Prefrontal Responses to Emotional Conflict in Patients
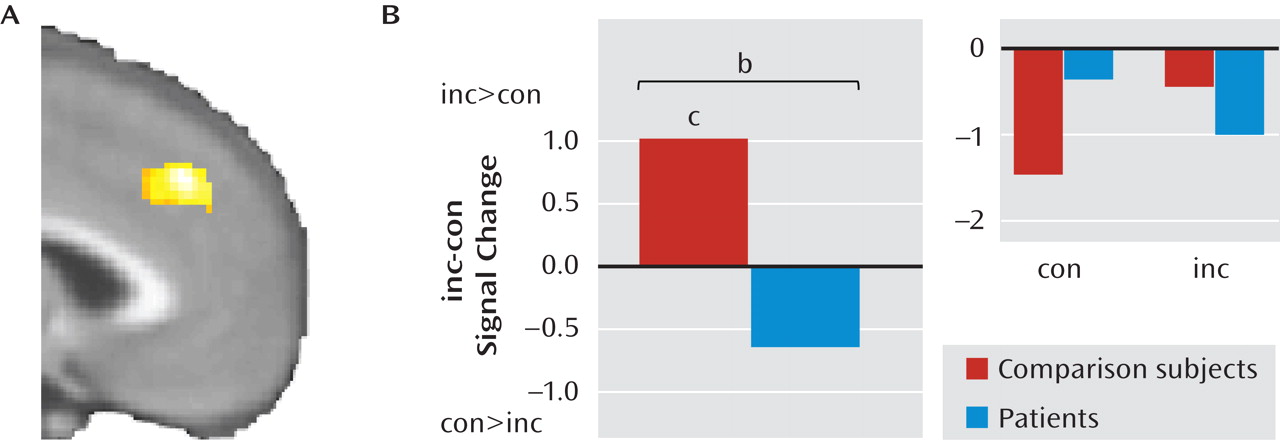
We first examined overall responses to emotional conflict (i.e., incongruent > congruent). As shown in
Figure 2A, healthy comparison subjects exhibited greater activation to emotional conflict than did patients with generalized anxiety disorder in the dorsomedial prefrontal cortex (x=0, y=36, z=38; z=3.96; d=1.22; 2,832 mm
3; x=6, y=44, z=34; z=3.33; d=1.14). This difference resulted from activation by emotional conflict within this cluster in comparison subjects (t=3.9, df=23, p=0.001; d=0.8) but not in patients (t=1.84, df=16, p>0.05; see Figure 2B). No group differences were observed in the pregenual cingulate or the amygdala.
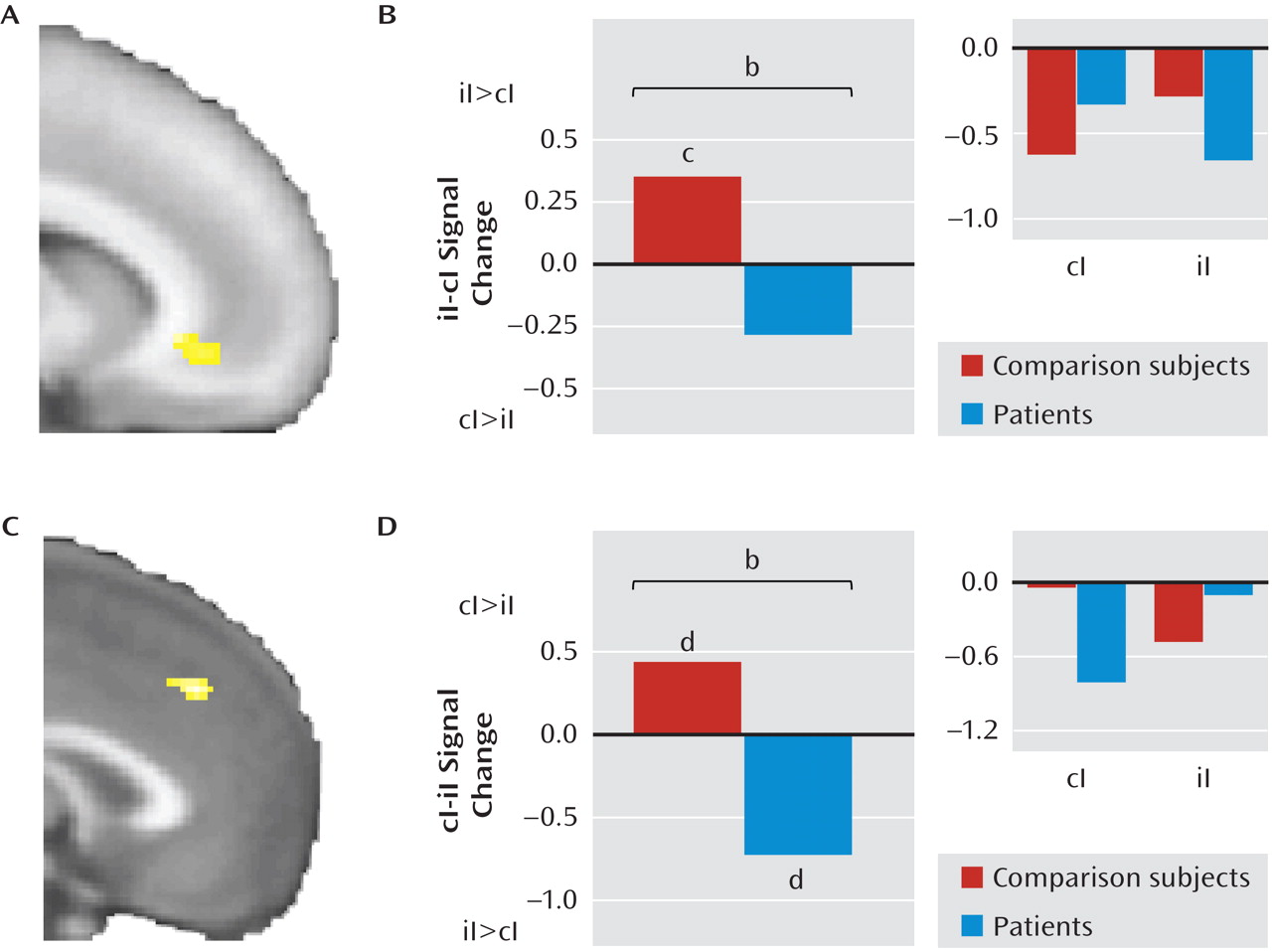
Next, we explored the neural correlates of group differences in emotional conflict adaptation, guided by our behavioral results. Based on our previous findings with the emotional conflict task in healthy volunteers (
15,
16), we examined the contrast of postincongruent incongruent trials minus postcongruent incongruent trials in the pregenual cingulate in patients and healthy comparison subjects and found a significant cluster (x=–12, y=32, z=–4; z=3.49; 376 mm
3; d=1.2; see
Figure 3A). Average signal within this cluster was extracted for each group to further describe the effect. As predicted, in this cluster, healthy comparison subjects had greater activity during postincongruent incongruent trials (t=3.34, df=23, p<0.005; d=0.68), whereas in patients no difference was observed (t=1.6, df=16, p>0.1; see Figure 3B).
Next, we examined the contrast of postcongruent incongruent trials minus postincongruent incongruent trials in the dorsomedial prefrontal cortex and amygdala in both groups and found a significant cluster in the dorsomedial prefrontal cortex (x=–1, y=36, z=38; z=3.26; 568 mm
3; d=1.15; see Figure 3C) but not in the amygdala. Extraction of average signal within this cluster revealed that the group difference was driven by the expected greater activity in postcongruent incongruent trials in healthy comparison subjects (t=2.36, df=23, p<0.05; d=0.48) and by the opposite effect in patients (t=2.66, df=16, p<0.05; d=0.64; see Figure 3D). Note that the inability of patients to decrease dorsomedial prefrontal activity in postincongruent incongruent trials paralleled patients' inability to improve reaction times during these trials compared to healthy comparison subjects. No group differences were observed in any of the regions of interest for the contrast of postcongruent congruent trials with postincongruent congruent trials. Finally, comparing across all trial types, we found significantly greater activation in patients than in comparison subjects in the left amygdala (x=–22, y=–2, z=–18; z=3.04; 232 mm
3; d=1.04). Using cytoarchitectonic probability maps of the basolateral, centromedial, and superficial amygdalar subregions (
47,
48), we found that 78% of this cluster corresponded to the superficial amygdala and 21.1% to the basolateral amygdala.
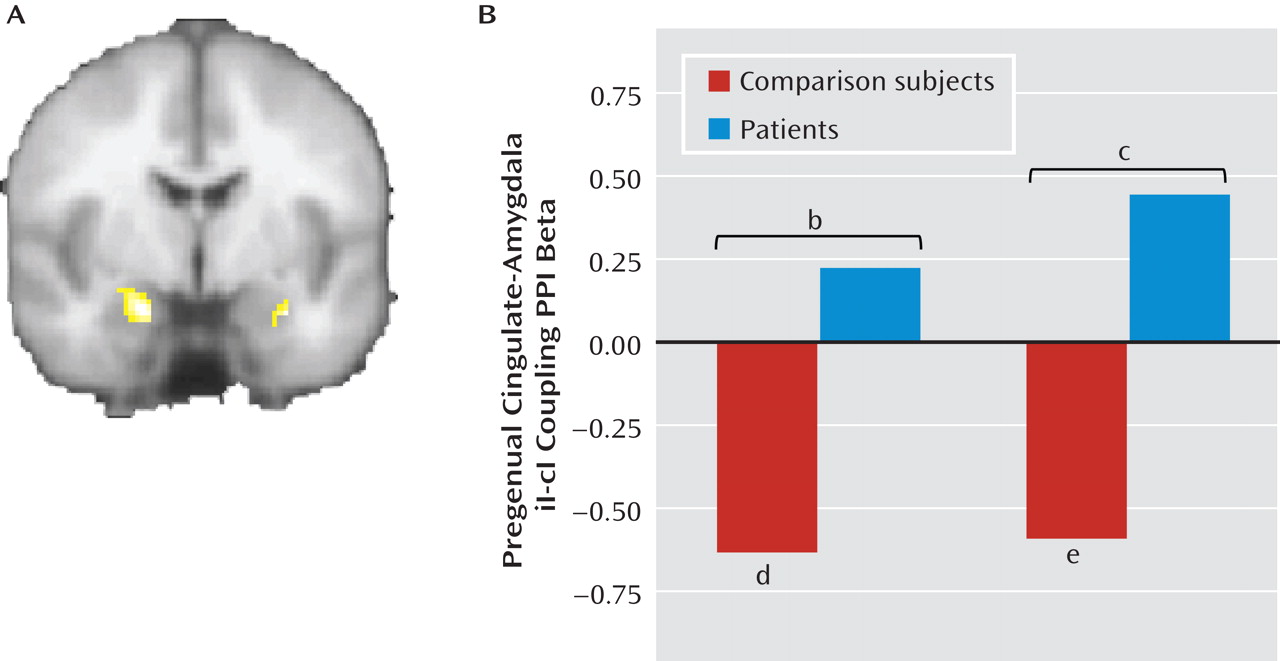
Absent Pregenual Cingulate-Amygdala Connectivity in Patients
We next examined differential functional connectivity between the pregenual cingulate and the amygdala during postincongruent incongruent trials compared with postcongruent incongruent trials using psychophysiologic interaction analyses, with the pregenual cingulate as the seed and the amygdala as the target, while controlling for task-related activations in both regions and task-nonspecific connectivity (
44). As shown in
Figure 4A, we found a significant group difference in both the left (x=–20, y=–4, z=–22; z=3.54; 536 mm
3; d=1.15) and right amygdala (x=30, y=–4, z=–22; z=3.4; 168 mm
3; d=1.15). Extraction of average connectivity strength within these clusters revealed that the group effect resulted from the predicted significant negative pregenual cingulate-amygdala connectivity in healthy comparison subjects during postincongruent incongruent trials, compared with postcongruent incongruent trials (left side: t=4.14, df=23, p<0.001; d=0.85; right side: t=3.08, df=23, p=0.005; d=0.63), but not in patients (see Figure 4B). We did not pursue further characterization of differential group cingulate-amygdala connectivity using effective connectivity methods such as dynamic causal modeling, as we had in a previous study of healthy volunteers (
16), since we did not think it would add significant new information beyond the result from the functional connectivity analysis above. Finally, we found that the majority of the left amygdala differential connectivity cluster was in the basolateral amygdala (55%), with 44.3% in the superficial amygdala and only 0.4% in the centromedial amygdala. One hundred percent of the right amygdala cluster was in the basolateral amygdala.
Additional Findings
We conducted several additional analyses to better understand the group differences reported above. First, for the patients, we correlated symptom scale scores with behavior and brain activity within the group difference clusters. We found that the impairment in emotional conflict adaptation was greatest, in terms of both reaction times and dorsomedial prefrontal modulation, for the most anxious patients (see the online data supplement). Second, we conducted multivariate pattern classification to determine whether behavior and brain activation could be used to determine participants' diagnostic group. Significant classification of patients and healthy comparison subjects could be achieved with both behavior and brain activation data, reaching 95% when whole-brain data were used (see the online data supplement).
Discussion
In this study, we investigated emotional conflict adaptation using a paradigm in which emotional processing is regulated spontaneously and in the absence of explicit instruction. We found that patients with generalized anxiety disorder were unable to adapt to emotional conflict through engagement of this regulatory process. By contrast, adaptation during congruent trials was similar in both groups, as was the overall reaction time interference due to emotional conflict, demonstrating the specificity of the deficit.
At the neural level, patients with generalized anxiety disorder failed to activate the pregenual cingulate and demonstrate negative top-down (
16) pregenual cingulate-amygdala connectivity during the regulation of emotional conflict. As in previous studies of emotion regulation (
27,
28), regulation-related changes in activity were seen in the context of overall task-independent medial prefrontal deactivation from an implicitly modeled baseline, and this deactivation did not differ between our groups (data not shown). Moreover, since the critical contrast involves only incongruent trials, the many processes that differ between incongruent and congruent stimuli are controlled for, as are nonspecific responses to task demands, leaving only the effect of previous trial conflict on processing of emotional conflict on the current trial.
We suggest that patients' failure to show the neural effects related to previous trial conflict accounts for their behavioral regulatory deficit during emotional conflict adaptation, in accordance with predictions made by the conflict monitoring hypothesis about brain activity during conflict adaptation—a cognitive model supported by an extensive neuroimaging literature (
17,
18,
20–
23,
25,
26). These conclusions are also consistent with emotion regulatory roles attributed to ventromedial prefrontal regions through connectivity with the amygdala in other studies (
27–
29,
49). Moreover, we recently found functional connectivity and structural evidence for an intra-amygdalar abnormality at a subregional level in generalized anxiety disorder (
12). Thus, it appears that patients have deficits both in activating relevant control regions (pregenual cingulate) and in the connectivity required for such regions to exert control over limbic structures. It is therefore interesting to speculate that the lateral prefrontal hyperactivation (
7,
9,
14) or increased connectivity with the amygdala (
12) previously reported in patients with generalized anxiety disorder may reflect the compensatory engagement of worry, an attention-demanding cognitive process, to regulate the effects of emotional stimuli in the absence of patients being otherwise able to recruit pregenual cingulate-based regulatory mechanisms.
Finally, there is controversy regarding overall emotional responsiveness in generalized anxiety disorder, indexed largely through activation in the amygdala. Pediatric studies have shown hyperactivity to negative emotional expression faces (
7,
8), and adult studies have shown either no difference (
10) or hypoactivation (
9) to similar stimuli, although one adult study showed nonspecifically exaggerated amygdalar reactivity to both negative emotional and neutral cues (
11). Consistent with the latter study, we found greater amygdalar activity in patients during both congruent and incongruent trials.
Our data also highlight an emerging theme in affective neuroscience, namely, that there are many ways by which emotional processing is regulated and that deficits in these functions contribute importantly to psychopathology. To date, the neurobiology underlying the regulation of emotional processing has been primarily studied by asking participants to deliberately alter their emotional responses to defined stimuli (i.e., "explicit" regulation) (
50). Much of the normal regulation of emotional processing, however, probably occurs in the absence of explicit effort (
51,
52). Far less is known about these "implicit" forms of regulation.
Based on the fact that behavioral performance in our task indicates the engagement of a mechanism for regulating emotional processing that occurs in the absence of specific regulation instructions, we and others have argued that emotional conflict adaptation is a type of implicit regulation of emotional processing (
51,
52). In this study, we provide direct behavioral evidence for this in healthy participants. The striking deficit in emotional conflict regulation in patients, in the context of otherwise intact task performance, provides the strongest evidence to date linking abnormalities in a defined form of implicit regulation and a type of psychopathology whose clinical presentation suggests emotion regulatory abnormalities.
Several limitations are important to note. First, we are unable to report on subjective ratings of emotion during emotional conflict adaptation, as asking participants to report on subjective emotional states might itself lead to emotion regulation (
53–
57). Thus, we inferred the effects of emotion from behavioral indices, such as reaction times, and patterns of brain activation. Second, although we focused in this study primarily on the neural effects we previously found to be specific to emotional conflict (
15), it would be useful in future experiments also to examine adaptation to nonemotional conflict. Finally, it is unknown whether medial prefrontal dysfunction during emotional conflict adaptation reflects a disorder-specific abnormality or a more general endophenotype of affective disorders, such as major depression. Nonetheless, the robust group differences seen at both the behavioral and neural levels suggest that the inability of patients to adapt to emotional conflict is an important aspect of the pathophysiology of generalized anxiety disorder—and potentially of other psychiatric disorders—and thus merits continued, deeper, study.
Acknowledgments
The authors thank James Gross, Tobias Egner, Raffael Kalisch, and Keith Sudheimer for helpful comments on the manuscript and Jennifer Keller for assistance with planning of the study.