Substantial efforts have been made in the past decade to elucidate the neural basis of major depressive disorder. Structural and functional neuroimaging studies of patients with depression have revealed a complex neuropathophysiology involving regional deficits in the limbic-thalamo-prefrontal and limbic-striatal-pallidal-thalamic systems (
1–
9). About 30% of patients do not respond to standard antidepressant treatment and are classified as having refractory depression, while those who respond have nonrefractory depression (
10). Little is known about how these two clinical subtypes differ at the neuronal level. We investigated the functional deficits in these two subtypes in the hope that noninvasive measurements might eventually make it possible to distinguish them at an early stage of clinical intervention. We recently (
1) identified regional cerebral perfusion differences between these groups: the refractory group showed reduced perfusion in prefrontal and thalamic areas, while the nonrefractory group showed reduced perfusion in left frontal areas and increased perfusion in limbic-striatal areas. The effects of these regional alterations in resting perfusion on systems-level disturbances in distributed brain networks are of course impossible to predict. There is increasing evidence that neural networks are disrupted in depression (
11–
15) as well as in other neuropsychiatric conditions, such as Alzheimer's disease (
16), schizophrenia (
17), and acute psychological trauma (
18). However, no study has yet determined whether patients with refractory and nonrefractory depression can be distinguished by differential functional integration within specific neural networks.
Resting-state functional connectivity MRI (fcMRI) (
19) has been increasingly used to investigate the integration of neural networks at a resting state when no task is performed (
20). Low-frequency (0.01–0.08 Hz) fluctuations of the blood-oxygen-level-dependent (BOLD) signal in the resting state are considered to be physiologically meaningful and related to spontaneous neural activity (
21). While task-based functional MRI (fMRI) studies can assess disturbances in functional connectivity when patients perform a particular task, assessment of resting-state connectivity has different and potentially broader significance, because it requires minimal patient compliance, can be obtained under anesthesia, and is well suited for translation into the clinical realm (
19). This technique has been successfully used to detect abnormal functional integration in major depressive disorder (
22).
Discussion
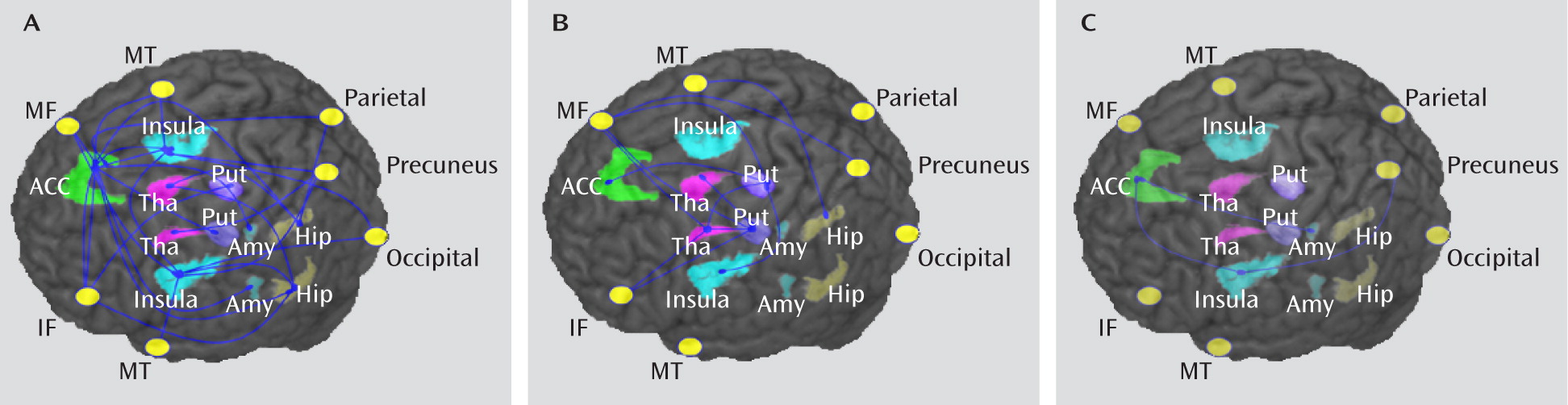
Using resting-state fMRI in a cohort of patients with well-characterized depression, studied before commencement of medication, we found altered functional connectivity mainly involving the frontal-subcortical circuits, which are strongly implicated in depression (
35). Furthermore, we observed differences in functional connectivity related to treatment responsiveness, with the nonrefractory group showing a decrease mainly in the limbic-striatal-pallidal-thalamic circuits (
Figure 1A), while the refractory group showed a decrease mainly in thalamo-cortical circuits (
Figure 1B).
Convergent evidence from functional brain imaging, therapeutics, and lesion studies suggests that depression is associated with dysfunction in several functionally integrated pathways (
36,
37). More specifically, a loss of top-down regulation, especially the loss of prefrontal cortex control over limbic regions, is thought to be at the root of the pathogenesis of emotional, behavioral, cognitive, and endocrine changes in depression (
38,
39). Consistent with this hypothesis, reduced fronto-limbic connectivity has been reported in both task (
40,
41) and resting-state (
42) fMRI studies in patients with depression, although results have been inconsistent, with reports of both increased and decreased connectivity. Our results confirmed the decrease in connectivity involving the prefrontal cortex in a cohort of 60 patients with depression. Furthermore, this decreased connectivity was more widespread in the group with nonrefractory depression than in the group with refractory depression.
The limbic system has widespread connections to the prefrontal cortex, amygdala, and thalamus (
43), and it plays a critical role in anxiety and depressive states (
44) in addition to its contribution to learning and memory. In the patients with nonrefractory depression, connectivity was decreased among distributed limbic areas, particularly in the anterior cingulate cortex and in the prefrontal and insula regions bilaterally (
Figure 1A). The same network of regions was identified in a recent meta-analysis of cortical-subcortical interactions in emotion processing (
45). Thus, it may be that decreased connectivity in this network underlies emotional dysregulation in these patients. The insula is thought to mediate interpretation of sensory information from the body (interoception) that contributes to emotional states (
46). Decreased connectivity in this circuit might therefore underlie such depressive symptoms as somatic complaints and negative bias in interpreting bodily feedback.
This decreased functional connectivity between prefrontal and limbic networks in the group with nonrefractory depression may also account for the inverse relationship of activation between prefrontal lobe and limbic regions reported in previous studies (
1,
40). Prefrontal cortical-limbic connectivity serves as an inhibitory link between those regions and is reduced in depression (
47). The consequent disinhibition might account for the overactivity of the limbic system in the group with nonrefractory depression. This in turn might stimulate the hypothalamic-pituitary-adrenal axis (
48,
49), and consequent glucocorticoid oversecretion could contribute to loss of frontal lobe integrity (
50). Such decreased connectivity has been reported to improve after 6 weeks of treatment with sertraline in responders (
51) and may have a genetic basis, for example, in the 5-HTTLPR allele (
52).
The finding of decreased functional connectivity in the group with nonrefractory depression relative to the group with refractory depression (
Figure 1C) is surprising, as one might have expected more impaired connectivity in the latter. However, this finding is not implausible in light of previous neuroimaging studies suggesting that functional alterations may be specifically present in nonrefractory patients. For example, in an investigation using arterial spin-labeling MRI (
1), we found that patients with nonrefractory depression but not those with refractory depression showed altered perfusion in the limbic system relative to healthy comparison subjects. One possibility is that alterations in nonrefractory patients are localized within the limbic system, which is also the target of standard antidepressants (
53), whereas alterations in refractory patients are expressed in a thalamo-cortical circuit, which may be less sensitive to antidepressant medication (
53). This would explain why pharmacological treatment is effective in only one clinical group even though both groups show altered brain functioning.
Despite this at-first-sight surprising result of direct comparison between the refractory and nonrefractory groups, the comparisons between each patient group and healthy comparison subjects appeared to suggest more disrupted alterations of functional connectivity in the refractory than in the nonrefractory group in prefrontal areas and in the thalamus areas bilaterally (
Figure 1B). This is consistent with results of previous studies (
54–
56) suggesting greater disruption within thalamo-frontal circuits in refractory depression relative to nonrefractory depression. For example, more severe frontal deficits are reported in patients with late-onset depression associated with frontal vascular disease (
57), who have higher rehospitalization rates and treatment resistance (
58). Also, therapeutic intervention targeting frontal areas has been reported to be useful in refractory patients (
59,
60) and to be correlated with clinical improvement (
61). Finally, increased thalamic metabolism has been reported in remitted depressed patients after tryptophan depletion but not after sham depletion (
62). Abnormal functional connectivity between thalamus and medial prefrontal regions has also been found to be associated with refractoriness (
22). These findings, together with the results of our investigation, suggest that refractory depression may be mainly associated with disrupted connectivity in thalamo-cortical circuits. This may partly explain why patients with refractory depression are refractory to standard antidepressants but respond well to treatments targeting frontal areas (
59–
61).
Several study limitations should be considered when interpreting these results. First, the data are cross-sectional; whether these altered neural networks change dynamically after therapy remains to be established in longitudinal studies. Second, patients were treated with a drug belonging to one of three different classes with heterogeneous pharmacological profiles. This heterogeneity limits the translational value of our results since the same patient may show a poor response to one drug class and a good response to another. Future studies aimed at informing clinical intervention will benefit from the investigation of a single drug, or at least drugs with the same pharmacological profile. Finally, the refractory group had a greater illness duration than the nonrefractory group. Although we used illness duration as a covariate in the statistical analysis, we cannot exclude the possibility that our results were influenced by this variable. Again, a longitudinal approach would allow examination of whether and how these altered neural networks change with the development of the illness.