Medications for attention deficit hyperactivity disorder (ADHD)—amphetamines, atomoxetine, and methylphenidate—are used by an estimated 1.5 million adults in the United States (
1). ADHD medications raise both blood pressure (<5 mm Hg) and heart rate (<7 bpm) (
2–
5). Given these effects, case reports of sudden death, stroke, and myocardial infarction have led to regulatory and public concern about the cardiovascular safety of these drugs (
1).
Our original goal in this study was to evaluate whether use of amphetamines, atomoxetine, or methylphenidate in adults is associated with higher rates of serious cardiovascular events than are seen in nonusers (
6). However, the size of the cohorts and the numbers of events in new users of amphetamines and atomoxetine were too small to yield reasonably precise measures of association. Therefore, in this analysis we focused on methylphenidate. The prespecified events of primary interest were 1) sudden death or ventricular arrhythmia, 2) stroke, 3) myocardial infarction, and 4) a composite endpoint of stroke or myocardial infarction (
6). Secondary events of interest were all-cause death and nonsuicide death. An additional aim of the study was to examine whether treatment factors (e.g., dosage and duration) and patient factors (e.g., age and preexisting cardiovascular conditions) influenced the relative hazards.
Method
Overview and Data Source
This was an investigator-initiated nonrandomized cohort study conducted using preexisting administrative data. The study's design and rationale (
6) and results from data on children and adolescents (
7) have been reported elsewhere. Briefly, data were used from two different U.S. sources: a five-state Medicaid database covering the period 1999–2003 and the 14-state HealthCore Integrated Research Database, a commercial insurance database, covering the period 2001–2006. These were the most current versions of these data available at the study's outset. In addition, Medicare data were obtained for all Medicaid-Medicare dual eligibles (
8).
The study was approved by the University of Pennsylvania's Committee on Studies Involving Human Beings, which granted waivers of the requirements for informed consent and Health Insurance Portability and Accountability Act authorization.
Study Subjects and Eligible Person-Time
All patients age 18 and older who filled a prescription for orally administered methylphenidate were identified. The prespecified primary cohort consisted of new users, defined as those who had at least 180 days of observation before the first observed methylphenidate prescription, and matched comparison subjects who did not use methylphenidate, amphetamines, or atomoxetine (“nonusers”). Each user was matched on data source, state, sex, and age in 6-year bands to as many as four nonusers who were at least 18 years old.
Follow-up started after the 180-day baseline period, during which baseline demographic and clinical characteristics were ascertained (see Table S1 in the data supplement that accompanies the online edition of this article). For new methylphenidate users, this was the 180-day period immediately preceding the first dispensed methylphenidate prescription. All person-time during an active methylphenidate prescription was included for the exposed group. The median duration of methylphenidate prescriptions according to the “days supply” field was 30 days, and the median time between fill dates of consecutive prescriptions was 34 days. We therefore made the assumption that the duration of a methylphenidate prescription was 30 days unless a subsequent prescription was dispensed before 30 days had passed. We performed sensitivity analyses assuming that each prescription lasted for a maximum of 60, 90, and 120 days to allow for patient nonadherence; the analyses also included events of interest that may have occurred shortly after cessation of therapy. The observation period ended with the earliest of 1) the first event of interest (for the analysis of that event), 2) the end of the last observed prescription period for exposed subjects, 3) the end of the study period, 4) disenrollment from the health care plan, or 5) death. HealthCore enrollees were censored when they turned 65, which is when Medicare eligibility begins for most people. In addition, users were censored when they switched to or added a different ADHD medication. In an additional sensitivity analysis, methylphenidate users were censored when they had a gap of more than 180 days between consecutive prescriptions.
Outcomes of Interest
The following prespecified outcomes were of primary interest:
1. Hospitalization or emergency department visit with a first-listed (principal) diagnosis of sudden cardiac death or ventricular arrhythmia (ICD-9 codes 427.1, 427.4, 427.41, 427.42, 427.5, 798.1, or 798.2)
2. Hospitalization with a first-listed diagnosis of stroke (ICD-9 codes 430, 431, 433.x1, 434 [excluding 434.x0], or 436)
3. Hospitalization with a first-listed diagnosis of myocardial infarction (ICD-9 code 410)
4. A composite of hospitalization with a first-listed diagnosis of either stroke or myocardial infarction
These operational definitions have been found to have high positive predictive values (85% for sudden death/ventricular arrhythmia [
9], >70% for stroke [
10–
14], and >90% for myocardial infarction [
15–
19]). A non-principal diagnosis for one of these diagnoses resulted in censoring for that outcome because non-principal diagnoses are likely to represent in-hospital events, which were not of interest in this study of outpatient medication use, yet which eliminate the individual's risk of experiencing an incident event.
Prespecified secondary outcomes were all-cause death and nonsuicide death (i.e., deaths excluding ICD-10 codes X60–X84 and Y87.0). The rationale for examining all-cause death was that most deaths in older adults have cardiovascular causes (
20). The rationale for evaluating nonsuicide death was the possibility that ADHD or its treatment may be linked to suicide. Deaths were ascertained using the Social Security Administration Death Master File and causes of death from the National Death Index.
Statistical Analysis
Study groups were compared and age-standardized incidence rates calculated using Stata, version 11 (StataCorp, College Station, Tex.). Proportional hazards regression (
21) was used to calculate minimally adjusted hazard ratios for methylphenidate compared with the matched unexposed group, with intracluster dependence within matched sets accounted for using a robust sandwich estimator (
22,
23). Because the low number of events did not permit adequate adjustment of potential confounders through multivariate models, a post hoc propensity score analysis was performed (
24) in which we included in the propensity score all baseline variables listed in the online data supplement and adjusted for propensity score decile.
Analyses to explore drug effect in high-risk subgroups were then performed based on age (<65 years compared with ≥65 years [in Medicaid enrollees only, since HealthCore enrollees ≥65 years were censored]), sex, and evidence of preexisting cardiovascular conditions. Because the age-stratified model included only Medicaid data, a new propensity score was created for these analyses.
Dosage and drug release characteristics (immediate or extended release) were evaluated to determine whether they influenced event rates. Because this model included only users, a new propensity score was created.
Finally, data were stratified based on follow-up time (1–180 days and 181–360 days) to determine whether differences in follow-up time influenced the results.
Proportional hazards analyses were performed using SAS, version 9.2 (SAS Institute, Cary, N.C.).
Results
A total of 43,999 new users of methylphenidate were identified and were matched to 175,955 nonusers.
Table 1 summarizes their baseline demographic and clinical characteristics. Methylphenidate users were more likely than nonusers to have preexisting cardiovascular conditions and psychiatric disorders and more likely to have received a variety of medications. The median follow-up time was 60 days (interquartile range, 30–149) for new users of methylphenidate and 538 days (interquartile range, 186–916) for nonusers.
Tables 2 and
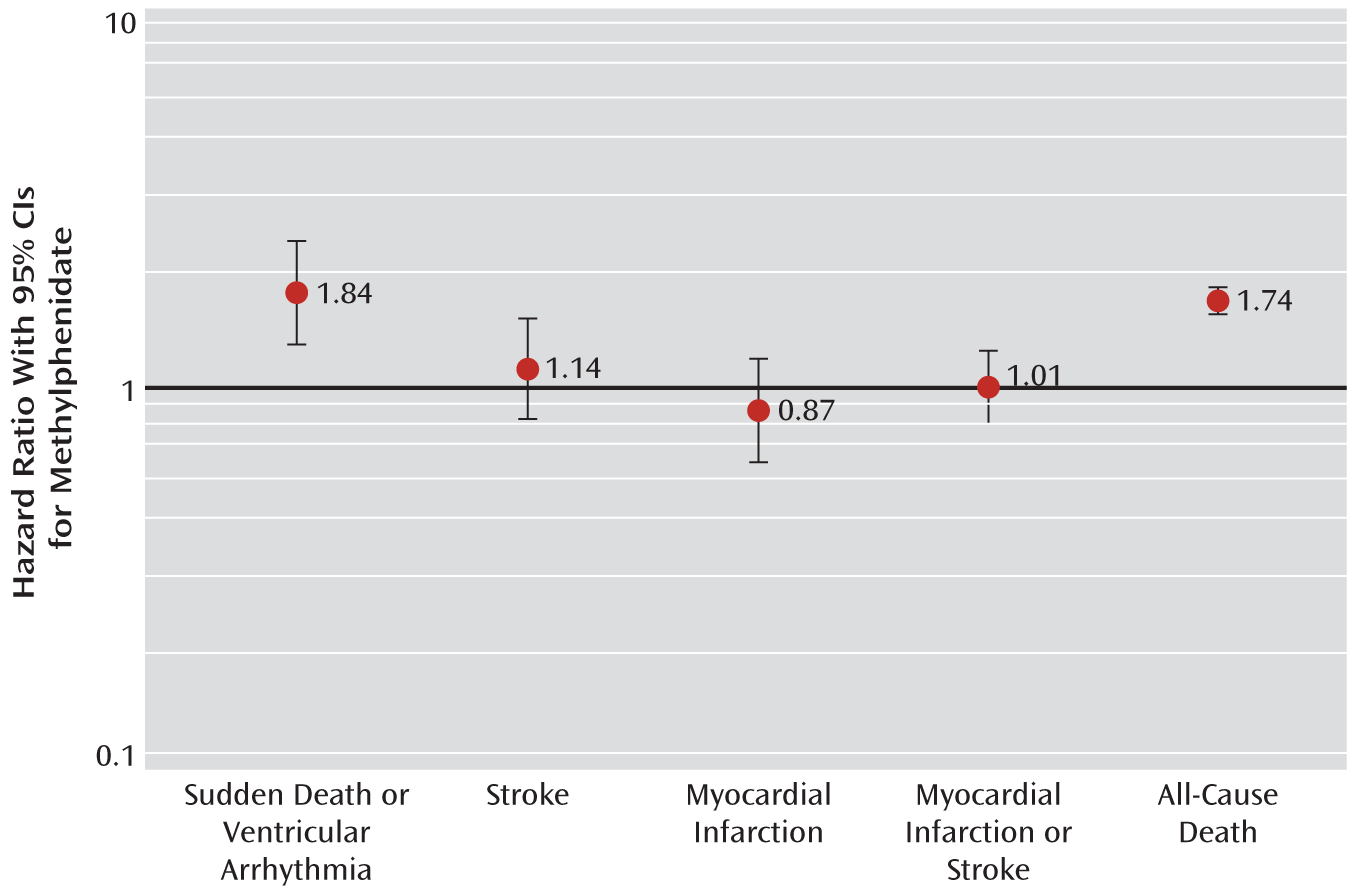
3 present the age-standardized incidence rates and minimally adjusted and propensity score-adjusted hazard ratios of new methylphenidate users compared with matched nonusers. Key hazard ratios are also presented in
Figure 1. For each outcome, propensity score adjustment attenuated the hazard ratios toward the null. The adjusted hazard ratio for sudden death/ventricular arrhythmia was 1.84 (95% CI=1.33–2.55). Adjusted hazard ratios for stroke, myocardial infarction, and stroke/myocardial infarction did not differ statistically from 1. For the secondary outcome of all-cause death, methylphenidate demonstrated a positive association (adjusted hazard ratio=1.74, 95% CI=1.60–1.89). The results of the analysis of nonsuicide deaths were nearly identical to those for all-cause death events (data not shown).
Several of the hazard ratios differed between the Medicaid and HealthCore populations. In particular, statistically significant differences were observed between the two data sources for myocardial infarction (Medicaid: adjusted hazard ratio=1.04, 95% CI=0.74–1.46; HealthCore: adjusted hazard ratio=0.34, 95% CI=0.13–0.91), stroke/myocardial infarction (Medicaid: adjusted hazard ratio=1.15, 95% CI=0.90–1.47; HealthCore: adjusted hazard ratio=0.55, 95% CI=0.30–1.01), and all-cause death (Medicaid: adjusted hazard ratio=1.59, 95% CI=1.46–1.73; HealthCore: adjusted hazard ratio=6.54, 95% CI=5.05–8.49). After exclusion of Medicaid enrollees over age 65 to correspond to the exclusion of this group in HealthCore, the differences in adjusted hazard ratios for myocardial infarction and stroke/myocardial infarction between the data sources were no longer statistically significant (p=0.13 and p=0.22, respectively). However, the adjusted hazard ratios for all-cause death remained statistically different between databases (p≤0.001; Medicaid: adjusted hazard ratio=1.32, 95% CI=1.10–1.58; HealthCore: adjusted hazard ratio=5.50, 95% CI=4.22–7.15).
We performed sensitivity analyses to examine the effects of several key study decisions. One such decision was to include individuals in Medicaid managed care plans. An analysis that excluded such enrollees produced similar results (data not shown), which suggests that incompleteness of claims as a result of enrollment in managed care did not meaningfully influence the results. Another study decision was to assume that methylphenidate prescriptions lasted 30 days unless a subsequent prescription was dispensed before 30 days had elapsed. Assuming instead that the maximum prescription duration was 60, 90, or 120 days resulted in similar or attenuated results (data not shown), except for all-cause death when the maximum prescription duration was assumed to be 90 days (adjusted hazard ratio=1.93, 95% CI=1.81–2.06) or 120 days (adjusted hazard ratio=1.95, 95% CI=1.83–2.08). An additional study decision was to include methylphenidate-exposed time regardless of the duration of the gap since the previous prescription. In an analysis that censored methylphenidate users after a gap of 180 days or more, the results did not differ from the primary results.
Table 4 presents results by dosage, type of drug release (immediate or extended release), and follow-up time. The median methylphenidate dosage among users with an event was 20 mg/day. There was no association between high dosage (defined as >20 mg/day) and sudden death/ventricular arrhythmia (high compared with low dosage, adjusted hazard ratio=1.07, 95% CI=0.60–1.90). However, there were unexpected inverse associations between high methylphenidate dosage and stroke (high compared with low dosage, adjusted hazard ratio=0.36, 95% CI=0.16–0.81), myocardial infarction (high compared with low dosage, adjusted hazard ratio=0.43, 95% CI=0.19–0.99), stroke/myocardial infarction (high compared with low dosage, adjusted hazard ratio=0.41, 95% CI=0.23–0.72), and all-cause death (high compared with low dosage, adjusted hazard ratio=0.50, 95% CI=0.40–0.63). Several extended-release formulations of methylphenidate are available, with different release characteristics. These formulations were neither positively nor negatively associated with sudden death/ventricular arrhythmia, stroke, myocardial infarction, or stroke/myocardial infarction. However, extended-release formulations were associated with a lower risk of all-cause death than immediate-release formulations (extended-release formulations, adjusted hazard ratio=0.40, 95% CI=0.30–0.53).
Table 4 also presents results stratified by duration of follow-up. The hazard ratios for all outcomes were numerically higher during the initial 180 days than during the subsequent 180 days, although the confidence intervals overlapped.
Table 5 presents results stratified by age, sex, and evidence of a baseline cardiovascular condition. None of the adjusted hazard ratios differed statistically by age or cardiovascular condition. The adjusted hazard ratio for myocardial infarction was lower in males than in females (adjusted hazard ratio=0.58, 95% CI=0.34–0.97 and adjusted hazard ratio=1.16, 95% CI=0.78–1.71, respectively; interaction p=0.03).
Discussion
There is substantial public health concern about the cardiovascular safety of ADHD medications in adults. This concern is due in part to the drugs' effects on heart rate and blood pressure and to their widespread and growing use in adults, including the elderly. In this study, initiation of methylphenidate was associated with nearly a doubling of the rate of sudden death or ventricular arrhythmia. If this association represented a true causal effect (for example, through a direct cardiac stimulatory effect), the incremental rate due to methylphenidate would be approximately 1 event per 1,000 person-years. In addition, we observed an adjusted hazard ratio of 1.7 for all-cause death among new methylphenidate users. However, the the lack of a dose-response relationship between methylphenidate and sudden death or ventricular arrhythmia does not support a causal relationship. Furthermore, the inverse relationships between dosage and the rates of stroke, myocardial infarction, the composite outcome of stroke and myocardial infarction, and all-cause death may suggest that lower dosages were prescribed to the frailest patients, who might have had a greater risk of all-cause death and sudden death—that is, the results may have been affected by unmeasured confounding.
At the time of writing, no published study has yet evaluated the rate of sudden death/arrhythmia during methylphenidate exposure in adults, and to our knowledge, only one case report of sudden death while taking methylphenidate has been published (
25). Holick et al. (
26) evaluated the cerebrovascular risk of atomoxetine and stimulants in adults and found a higher rate of transient ischemic attack (hazard ratio=3.44, 95% CI=1.13–10.60) but not of cerebrovascular accident (hazard ratio=0.71, 95% CI=0.34–1.47) in adults initiating stimulants compared with the general population. As our results do not suggest an increased stroke rate for methylphenidate, they agree with Holick and colleagues' results on cerebrovascular accident. A case-control study using U.S. mortality data found that individuals 7–19 years of age who died suddenly had a significantly higher odds of having used stimulants compared with individuals who died in motor vehicle crashes (odds ratio=7.4, 95% CI=1.4–74.9) (
27). This result was not replicated in our previously reported results from this study in children ages 3–17 (
8).
Because the study was nonrandomized, an important limitation is the potential for unmeasured confounding. Administrative data do not include many factors that might be examined as potential confounders, including smoking, blood pressure, nonprescribed aspirin use, substance misuse, and level of physical activity. Indeed, our results suggest that preexisting cardiovascular disease and treatment for a cardiovascular disease were more prevalent in methylphenidate users than in nonusers. If this is also true of unmeasured cardiovascular risk factors, then the associations reported here might be artificially elevated.
A second limitation is the potential for information bias, which could have occurred if the study outcomes of interest did not come to medical attention or were diagnosed preferentially in one group compared with the other. However, given the severity and high positive predictive value of the outcomes of interest, this seems unlikely for the primary endpoints, and death should be completely ascertained. To further address this concern, all-cause death was examined as a secondary outcome, which in the elderly is most commonly due to cardiovascular causes (
20). In the elderly, methylphenidate was associated with an adjusted hazard ratio of 1.54 (95% CI=1.41–1.70) for all-cause death.
A third limitation is the limited duration of follow-up. In particular, the median follow-up for methylphenidate was only 60 days. Nonetheless, the second 180 days of methylphenidate therapy was consistently associated with numerically lower event rates than the first 180 days, which could be due to depletion of susceptibles. A fourth limitation is limited precision in the stratified analyses because of the low event rate. Furthermore, some of the statistically significant results of the stratified analyses might have been due to multiple comparisons. Finally, the difference in the hazard ratio for death between Medicaid and HealthCore was not expected and is difficult to interpret.
Acknowledgments
The authors acknowledge Gina Chang, Maxine Fisher, Qing Liu, Tracey Quimbo, Keith Rodgers, Daohang Sha, and Qufei Wu for obtaining data, for programming, and/or for doing statistical analyses. They also thank Gerrie Barosso for her help in obtaining and using Centers for Medicare and Medicaid Services data.