A number of adverse exposures in utero or in the neonatal period have been associated with the later development of schizophrenia and other nonaffective psychoses. These include exposures to maternal malnutrition or infections and complications of pregnancy and birth (
1). The mechanisms underlying these associations are unknown, and a variety of hypotheses have been tested experimentally. For example, animal studies suggest that activation of maternal immune responses during fetal development can cause behavioral deficits involving both cognitive and emotional domains in adult offspring (
2). Indeed, reports of an elevated risk for schizophrenia among offspring of women with high blood levels of interleukin-8 (
3) or tumor necrosis factor-α (
4) during pregnancy support this notion. A register-based study by Eaton et al. (
5) indicated that chronic inflammatory or autoimmune conditions, such as celiac disease, are more common among parents of patients with schizophrenia than among comparison parents.
Recent studies have illustrated the usefulness of archival dried blood samples collected prospectively during neonatal screening for metabolic disorders (e.g., phenylketonuria) as a source of information on early life exposures that may be associated with diseases that have an adult onset. Such studies have reported an association between high levels of immunoglobulin G (IgG) directed at the protozoan
Toxoplasma gondii (
13) and at herpes simplex virus type 2 (
14) and the future development of schizophrenia. IgG is actively transported across the placenta during the later stages of pregnancy to provide passive immunization of the fetus (
15), and hence such antibodies reflect maternal exposures and immune responses to specific antigens. Using dried blood spots obtained from newborns, we investigated whether levels of IgG directed at food-derived antigens, namely, gliadin (a component of wheat gluten) and casein (a bovine milk protein), were associated with a later diagnosis of a nonaffective psychosis.
Method
The study population consisted of individuals born in Sweden between 1975 and 1985. Case subjects were diagnosed with nonaffective psychoses (see diagnoses below) as inpatients between 1987 and 2003 or as outpatients between 1997 and 2003, in Stockholm County Council. Inpatient data were extracted from the National Patient Register, and outpatient data from a local psychiatric health care registration system (the Psychiatric Care System) used in Stockholm County Council. To be included in the study, case subjects had to be alive (20 were deceased), be residents of Sweden (two had emigrated), and have a registered address (24 did not). By these criteria, 739 eligible case subjects were identified after verification of diagnoses in medical records at the different psychiatric clinics by two trained psychiatric nurses, and all were contacted. Of these, 337 (45.6%) did not respond to contact and 139 (18.8%) declined to participate, leaving a final sample of 263 case subjects (participation rate, 35.6%).
The comparison subjects were selected from a population-based register at the National Board of Health and Welfare and matched for sex, date of birth, birth hospital, and municipality. Letters were sent to potential participants with the aim of recruiting four matched comparison subjects per case subject. To be included in the study, comparison subjects could not have a history of inpatient psychiatric admission (according to the National Patient Register), had to be alive (two of those initially selected were deceased), had to be residents of Sweden (16 of those initially selected had emigrated), and had to have a registered address (38 of those initially selected did not). A total of 1,553 eligible subjects were identified. Of these, 660 (42.5%) did not respond to contact and 244 (15.7%) declined to participate, leaving 649 comparison subjects (participation rate, 41.8%). Thus, significantly more comparison subjects than case subjects consented to participate (χ2=8.0, df=1, p=0.005). Given the number of potential comparison subjects who declined to participate, the majority of case subjects (79.2%) had fewer than four matched comparison subjects.
Diagnoses
Nonaffective psychoses were defined according to DSM-IV, ICD-9, or ICD-10 codes. For schizophrenia, we used DSM-IV codes 295.x, excluding 295.7; ICD-9 codes 295.x, excluding F and H; and ICD-10 code F20. For other nonaffective psychoses, we used DSM-IV code 295.7 for schizoaffective disorders, code 297.1 for persistent delusional disorders, code 297.3 for induced delusional disorder, code 298.8 for acute and transient psychotic disorders, code 298.9 for unspecified nonorganic psychosis, and code 301.22 for schizotypal disorder; we used ICD-9 code 295F for schizotypal disorder, code 295H for schizoaffective disorders, code 297 for delusional disorders, and code 298 excluding A and B for reactive psychoses (excluding depressive and manic psychoses); we used ICD-10 code F21 for schizotypal disorder, code F22 for persistent delusional disorders, code F23 for acute and transient psychotic disorders, code F24 for induced delusional disorder, code F25 for schizoaffective disorders, code F28 for other nonorganic psychotic disorders, and code F29 for unspecified nonorganic psychosis.
Data From the Swedish Medical Birth Register
The Swedish Medical Birth Register, which was initiated in 1973, includes information for all deliveries in Sweden as well as data from the prenatal and neonatal periods. From the Register, we collected information on gestational age, weight, and length at birth for offspring, as well as data on maternal immigration and maternal age at delivery.
Blood Spots
In Sweden, blood is collected on a filter from all newborns in a screening program for early detection of metabolic diseases (e.g., phenylketonuria). Since 1975, these dried blood filters have been stored at Karolinska University Hospital, Huddinge. For this study, one blood spot from each consenting participant was excised from the filter and transferred to an individual resealable plastic bag. Filters were retrieved for 874 (252 case subjects and 622 comparison subjects) of the 912 (95.8%) individuals who consented to the study, with no significant difference between the two groups. Of these, 211 case subjects and 553 comparison subjects were matched sets.
Processing and Analyses
A disk 3.2 mm in diameter was punched from each blood spot and distributed into deep 96-well plates sealed with AxyMats (Axygen, Union City, Calif.). Proteins were eluted from the filter paper by incubation in 100 μL of phosphate-buffered saline for 1 hour at 37°C. Levels of IgG antibodies directed at whole casein or gliadin in the eluates were measured by solid-phase enzyme-linked immunosorbent assay, as previously described (
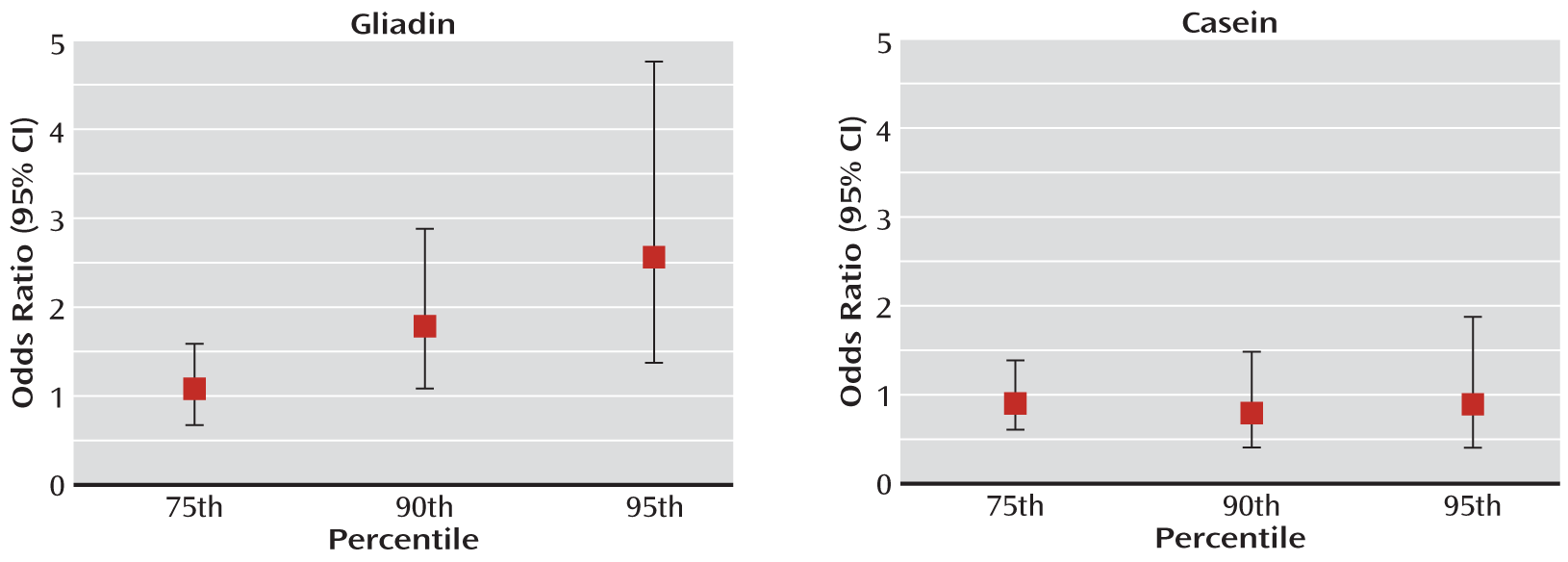
10,
16). The levels of antibodies were analyzed as the 75th, 90th, and 95th percentiles of reactivity, as previously described (
10); the cutoff points were based on the distribution among comparison subjects. During processing, all personnel were blind to case-control status of the filters.
Statistical Analysis
Conditional logistic regression for matched data were used in this case-control study. Potential confounding by maternal age, immigration status, or other factors related to pregnancy or birth, not matched for, was considered in these analyses. Logistic regression was used to analyze the association between potential confounders and high levels of gliadin and casein. SAS, version 9.1 (SAS Institute, Cary, N.C.), was used in all statistical analyses.
Approval
The study was approved by the regional research ethics committee at Karolinska Institute, Stockholm. After receiving a complete description of the study, participants provided written informed consent.
Discussion
We report on an elevated risk for developing nonaffective psychoses associated with high levels of antibodies directed at gliadin, a component of wheat, in dried blood spots obtained during the neonatal period. No such risk was associated with high levels of antibodies directed at casein, a dietary antigen abundant in cow's milk. The association between these antibodies in perinatal blood samples and the subsequent development of psychosis has not been previously investigated.
Antibodies of the IgG class detected in the neonatal blood samples are predominantly derived from the maternal circulation and transferred across the placenta during pregnancy (
15). These antibodies are therefore likely to represent maternal reactivity to gliadin and thus suggest that mothers who produce high levels of these antibodies during pregnancy give birth to children who have an elevated risk of developing a nonaffective psychosis later in life. We did not find an association with antibodies to casein, suggesting that the risk is not associated with an overall increase in antibodies to food antigens. While maternal age and immigration were associated with an elevated risk for nonaffective psychosis in the offspring, neither these factors nor mode of delivery contributed to the association between high IgG levels and risk of psychosis in the offspring.
IgG anti-gliadin antibodies are often, but not exclusively (see below), observed in individuals with celiac disease. Celiac disease is a rare condition affecting approximately 1% of the population. It is characterized by an autoimmune enteropathy triggered by ingestion of gluten (
21). Several studies have reported that untreated maternal celiac disease (but not treated disease) increases the risk for adverse pregnancy outcomes, such as low birth weight, intrauterine growth restriction, and prematurity (
18–
20). The mechanisms underlying these associations are not known with certainty but are believed to involve both autoantibodies affecting placental function (
22,
23) and maternal malnutrition due to intestinal malabsorption (
19). While the adverse birth outcomes associated with untreated maternal celiac disease are also risk factors for a later diagnosis of psychosis (
24,
25), we found no evidence for restricted fetal growth (as measured by birth weight and ponderal index) being involved in the association between high levels of IgG anti-gliadin antibodies and risk for nonaffective psychosis in the offspring.
Since the bulk of the IgG present in the neonate at birth is transferred across the placenta during the last month of pregnancy, difference in gestational length is another factor that can lead to differences in levels of maternal IgG detected in the neonatal circulation. Gestational age did not, however, differ significantly across levels of gliadin antibodies among case or comparison subjects in our sample. Taken together, these observations suggest that fetal malnutrition due to subclinical maternal celiac disease or other causes is unlikely to explain the association between maternal levels of anti-gliadin IgG and the development of psychosis in offspring.
Other mechanisms explaining the association between maternal antibodies to gliadin and the subsequent development of psychiatric disorders in offspring need to be considered. Tentatively, our findings may represent common genetic factors for increased gluten sensitivity and celiac disease and psychotic disorders. Along these lines, Eaton et al. (
5), using Danish population registers, reported an overrepresentation of celiac disease among patients with schizophrenia as well as among their parents. Elevated levels of markers of celiac disease have also been observed in patients with psychiatric disorders (
26–
29). It should be noted, though, that while levels of IgG directed at gliadin are higher in these patients than in comparison subjects, their immune response to gliadin often appears to differ from that observed in patients with celiac disease, and they do not appear to generate antibodies against autoantigens commonly observed in patients with celiac disease (
10,
29,
30). Moreover, genome-wide association studies of schizophrenia indicate that the HLA-DQA*0501 and DQB*0201 alleles, present in almost 90% of patients with celiac disease (
31), are not overrepresented among schizophrenia patients (
10) and even appear to be associated with a lower risk for schizophrenia (
32). Studies of non-HLA genes have so far failed to identify common genetic risk factors for celiac disease and schizophrenia (
33). The existence of genetic factors common to elevated levels of IgG anti-gliadin antibodies and nonaffective psychoses, other than schizophrenia, remains to be determined.
Elevated levels of IgG anti-gliadin antibodies are also observed in individuals who do not meet the criteria for celiac disease. Gluten sensitivity is a clinical entity used to describe individuals who do not exhibit the autoimmune component of celiac disease but who experience distress following gluten ingestion and who have heightened levels of IgG (and IgA) anti-gliadin antibodies (
34). Moreover, studies from the United Kingdom (
35) and Sweden (
36) report that 10%–20% of the general population have IgG antibodies to gliadin in the absence of other markers of celiac disease, which agrees well with our findings of a risk confined to the highest decile. Therefore, to effectively confirm or rule out celiac disease in the mothers, serological tests more specific for celiac disease, such as measurements of maternal IgA antibodies to gliadin and antibodies directed at tissue transglutaminase, should be performed in future studies when maternal plasma is available (
17). Insufficient material and the fact that maternal IgA antibodies (the measurement of which is required for the full assessment of celiac disease) do not readily cross the placenta prevented us from further characterizing the maternal response to gliadin in the present study.
Another mechanism potentially linking maternal anti-gliadin reactivity with the later development of psychosis in offspring involves maternal inflammation. Celiac disease is associated with chronic inflammation of the small intestine (
37). Heightened sensitivity to gluten can also be observed in patients with functional gastrointestinal disorders caused by infections or other causes (
38). Moreover, patients with gluten sensitivity appear to exhibit a strong activation of the innate immune response (
34). Therefore, it is possible that mothers with high levels of anti-gliadin antibodies suffer from some degree of inflammation (
39), which can affect the developing fetus. As mentioned earlier, several experimental reports support this notion (
2). Direct evidence for an association between elevated maternal levels of inflammatory mediators and the development of psychosis in offspring has also been reported (
3,
4).
Finally, the association between maternal gliadin antibodies and the development of psychosis in offspring can potentially be explained by maternal diet. Indeed, ingested gluten has been proposed to have direct effects on neuronal function, a thesis supported by both clinical (
40,
41) and experimental studies (
42). However, the potential effects of exposure to dietary gluten during early life have not been investigated.
Strengths and Limitations
This study was based on archived samples from the neonatal period. These samples provide an invaluable source of information on early life exposures associated with illnesses later in life. Some limitations should be noted, however. The numbers of case and comparison subjects were limited because of fairly high losses to follow-up, which were somewhat higher among case subjects than among comparison subjects. This is not unexpected given that the case subjects suffer from a serious psychiatric condition. Consequently, there may be a selection bias in this study, which should be kept in mind when considering the generalizability of the results. Insofar as we can assess this bias, it should be noted that case subjects who consented to the study did not differ significantly in age, sex, or diagnosis from those who did not.
Another limitation is that the diagnoses in the study are register-based. Diagnoses from the National Patient Register (
43,
44) as well as the Psychiatric Care System (
43,
44) have, however, been validated and have proven to be of excellent quality. Moreover, in our consent procedure, a verification of registered diagnoses was required before contact with case subjects, allowing us to exclude individuals with erroneous register-based diagnoses. While case and comparison subjects were carefully matched on a number of demographic parameters, maternal factors that were not available to us in this study (e.g., medical diagnoses and dietary habits) could potentially modify or confound the associations observed.