Deep brain stimulation (DBS) is a reversible therapeutic technique that involves the bilateral placement of electrodes at specific neuroanatomical sites to deliver continuous stimulation from a subcutaneously implanted pulse generator. It has recently emerged as an important treatment for a number of movement disorders, including severe and medically intractable Tourette's syndrome. The pathophysiology of Tourette's syndrome is widely thought to result from a defect in the cortical-basal ganglia-thalamo-cortical neuronal circuit, and this has been the target of modulation with DBS for the control of tics.
The literature on DBS for Tourette's syndrome has recently been summarized (
1) and generally comprises single case reports or reports on a small series of patients (
2). To date, there have been reports of 63 patients having received DBS in 19 centers worldwide, with 59 patients (93.6%) showing moderate to marked improvement in tic severity (
1). Nine different brain targets in the thalamus, pallidum, ventral caudate, and anterior internal capsule have been stimulated (
2). The largest cohort was in Italy (
3), with the targets primarily being the centromedian-parafascicular and ventralis oralis complex of the thalamus. Of the 36 patients treated, follow-up data were reported for 30, and significant improvement was noted on all administered scales. Stimulation of the centromedian nucleus-substantia periventricularis-nucleus ventor-oralis crosspoint was recently subjected to a double-blind, randomized crossover trial in six patients who experienced substantial improvement (37% improved score on the Yale Global Tic Severity Scale) when stimulation was on compared with when it was off (
4). In another controlled, double-blind, randomized crossover study of three patients (
5), the authors demonstrated that bilateral stimulation of the globus pallidus interna produced a more favorable and persistent improvement in tic severity (an average of 78% reduction in tics) than stimulation in the centromedian-parafascicular thalamus (an average of 45% reduction in tics). This is understandable, as the globus pallidus interna is the primary output nucleus of the basal ganglia to the thalamus, and its stimulation modulates the basal ganglia-thalamo-cortical loop (
6). Furthermore, a recent case series of five patients (
7) demonstrated superior improvement in tic severity with stimulation in the anteromedial compared with the posterolateral globus pallidus interna (improvements of 38% and 20%, respectively) in Yale Global Tic Severity Scale score (
8). No neuropsychological, psychiatric, or other long-term adverse effects were observed.
In this study, we assessed a series of 11 patients with severe, treatment-refractory Tourette's syndrome who underwent DBS of the anteromedial globus pallidus interna bilaterally. We evaluated the effect of DBS on tic severity and on comorbid disorders, including obsessive-compulsive disorder (OCD) and major depression, and we examined the effect on quality of life and functional outcomes.
Method
Patients were recruited from the Movement Disorders Clinic of St. Andrew's War Memorial Hospital in Brisbane, Queensland, Australia. The selection criteria for DBS were a DSM-IV-TR diagnosis of Tourette's syndrome, documented nonresponse to multiple pharmacotherapeutic agents of known efficacy in global tic severity, Tourette's symptoms severe and disabling enough to substantially reduce the patient's quality of life, tic symptoms not considered secondary to another medical condition, medical suitability to undergo surgery, competency to provide informed consent, and age at least 18 years. The patients were examined independently by a psychiatrist, a neurologist, and a neurosurgeon, all of whom had to agree for the implantation procedure to proceed.
Eleven patients met criteria for DBS and were treated between September 2008 and December 2010. Institutional ethics approval was obtained from the hospital review board of St. Andrew's War Memorial Hospital. After participants received a complete study description, they gave written informed consent.
Before surgery, each patient completed a series of questionnaires. The primary outcome measure was the Yale Global Tic Severity Scale (
8), which is a validated, standardized rating scale for motor and phonic tics, yielding a total possible tic severity score of 50 and a total possible functional tic-related impairment score of 50, or a final total possible score of 100. Higher scores indicate a greater number and severity of tics and a higher degree of functional impairment. Secondary outcome measures included the Yale-Brown Obsessive Compulsive Scale (YBOCS;
9), the Hamilton Depression Rating Scale (HAM-D;
10), the Gilles de la Tourette Syndrome–Quality of Life Scale (
11), and the Global Assessment of Functioning Scale (GAF;
12).
Each patient underwent implantation of a Medtronic multiprogrammable quadripolar DBS system (Medtronic, Inc., Minneapolis). Patients received either the Soletra system (model 7426), which has a separate battery for each side, or the Activa PC device (model 37601), which has a single battery for both sides.
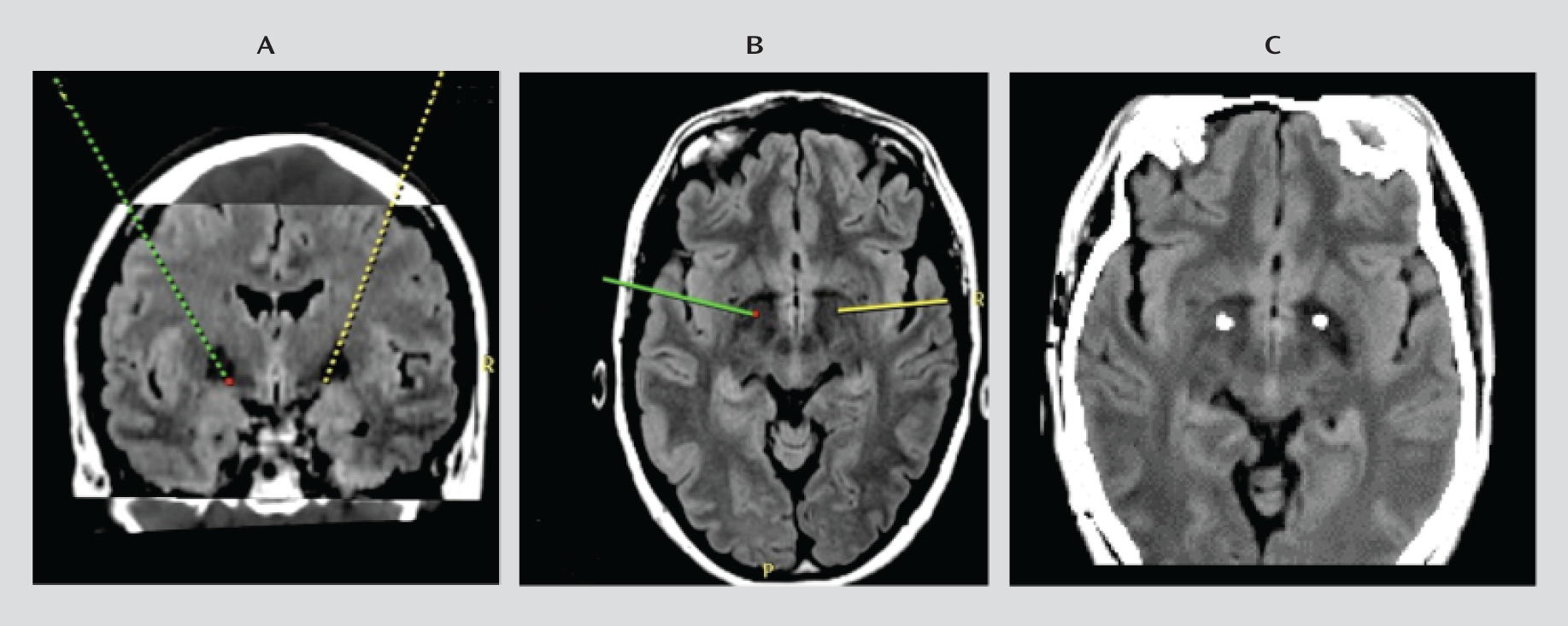
Surgery was performed under general anesthesia (propofol) using Cosman-Roberts-Wells frame-based stereotaxy with direct target localization and microelectrode recording. Before surgery, thin CT slices (1 mm) were obtained and merged with 3-T General Electric MRI T
1 and fluid-attenuated inversion recovery (FLAIR) sequences. The patients were then placed on a Medtronic Stealth surgical planning station. The target was directly visualized (see
Figure 1, panels A and B, and Figure S1 in the data supplement that accompanies the online edition of this article), and the coordinates were determined. While the patient was under general anesthesia, microelectrode recordings were obtained to identify the caudal border of the globus pallidus interna. The recording electrode was removed and a permanent Medtronic 3389 lead was placed, with active contacts spanning the globus pallidus interna. The Medtronic impulse generator was placed in the subclavicular region. A postoperative brain CT scan was undertaken, and the results were superimposed on the preoperative MRI scan to confirm correct lead positioning (
Figure 1C). DBS commenced in the immediate postoperative period when the patient had recovered from the anesthetic, and all patients were closely monitored in the intensive care unit for postoperative complications.
Initial stimulation parameters were set at 1.0 V bilaterally, with pulse width of 60 μsec and a frequency of 130 Hz and with unipolar activation of the caudal contacts, usually contact 0 or 1. Stimulation parameters were adjusted at follow-up visits depending on residual tic severity and the presence of side effects. The most commonly used increments were 0.5 V every second day. The final stimulation parameters for the patients are documented in Table S1 in the online data supplement. Patients were discharged from the hospital on day 5.
Patients were followed up weekly for 1 month, monthly for 3 months, and then quarterly. Stimulation parameters were adjusted as required over the course of follow-up. Rating scale assessments were repeated 1 month after surgery and at final follow-up (mean=14 months, range=4–30 months). At these time points, information was also obtained on any adverse effects. Assessments were performed by two authors (E.C. and P.S.S.) independently of the surgical team (P.S., T.C., and K.O.).
Data were analyzed using PASW Statistics, version 19 (SPSS, IBM Corp., Armonk, N.Y.). The nonparametric Friedman test was used to test for the statistical significance of variation of measures across three time points. Paired comparisons between measures before DBS and at two post-DBS time points were performed using the Wilcoxon signed-ranks test. Because we performed multiple tests, an alpha of 0.01 was considered statistically significant.
Results
Sample Characteristics
Individual patient characteristics are listed in
Table 1. The mean age was 33 years (range=18–50 years), and the majority of patients were men (73%, N=8). All had severe disabling tics and had tried between three and six medications before being considered for DBS. Most of the patients described low self-esteem and embarrassment as a consequence of tics. The youngest patient, who was 18 years old, had very severe Tourette's syndrome with associated self-injurious behavior, coprolalia, damage to property, and harm to others. She also had OCD and attentional difficulties, was unable to attend school, and had been hospitalized on a number of occasions, requiring 24-hour monitoring for self-injurious tics. She had been hospitalized for 2 years before the DBS procedure, and she had sustained a number of mild head injuries as a consequence of her Tourette's syndrome. All patients had received multiple treatments, including pharmacotherapy and cognitive and behavioral therapies, for a number of years before DBS. Patients were followed up clinically for between 4 and 30 months.
Treatment Response
Ten of the 11 patients (91%) reported a positive response to DBS, with reductions in tic numbers, severity, frequency, and intensity, and response occurred within 1 to 3 days of the surgery in nine of the patients. Patient 11 reported an increase in tic severity as well as adverse effects, and he subsequently switched off the stimulator after 3 months of DBS. Treatment response was defined as a reduction in the total score on the Yale Global Tic Severity Scale by more than 50%. Using this definition, six patients (54.5%) responded to DBS, although four others also had a meaningful improvement, as evidenced by change in scores on the Gilles de la Tourette Syndrome–Quality of Life Scale and the GAF (see Table S2 in the online data supplement).
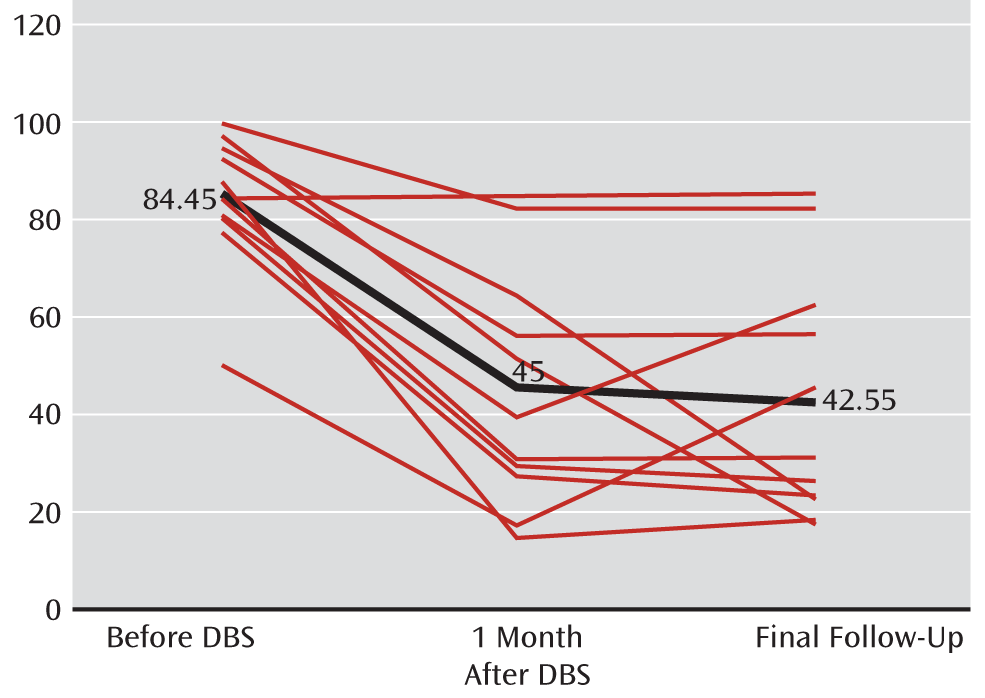
Overall, scores from all four components of the Yale Global Tic Severity Scale improved significantly after DBS compared with baseline, as did all secondary outcome measures. There was a 48% reduction in motor tics and a 56.5% reduction in phonic tics at final follow-up. When analyzing data from the 10 patients who had a positive response to DBS, both motor and phonic tics were reduced by more than 50% at final follow-up (53% and 62%, respectively). Overall, there was a reduction in the mean total score on the Yale Global Tic Severity Scale from 84.45 before surgery to 42.55 at 3-month follow-up, representing a 49.6% reduction in total tic severity (z=2.805, p=≤0.002). Results are summarized in
Table 2, and results for individual patients are presented in Tables S2 and S3 in the online data supplement. The total scores for individual patients on the Yale Global Tic Severity Scale are presented in
Figure 2. These scores did not change significantly from 1 to 3 months after DBS, but scores on the HAM-D were further improved during this period.
The patient who switched off the stimulator after 3 months of treatment reported many somatic complaints when we adjusted the stimulation parameters, such as feeling “overheated” and “fuzzy” in the head, although results of a physical examination at the time were normal. A postoperative MRI scan confirmed the correct placement of the electrodes, and stimulation parameters were increased to 3 V with pulse width at 60 msec and a frequency of 160 Hz before turning off the stimulator. During the time the stimulator was switched on, the patient's score on the HAM-D increased from 7 to 9, and his scores on the Gilles de la Tourette Syndrome–Quality of Life Scale and the GAF decreased. After the stimulator was switched off, the assessments were repeated, and scores were unchanged from baseline.
Of the 11 patients in the study, nine reported obsessive-compulsive symptoms before DBS. Overall, a significant reduction in the mean YBOCS score from 15.82 to 6.55 (59%) was observed following DBS surgery. There was also an overall reduction in mean HAM-D score from 16.45 to 4.27 (74%). Apart from patient 11, the improvement in HAM-D was acute in onset (i.e., in less than 1 month), but unlike the total score on the Yale Global Tic Severity Scale, the HAM-D scores showed further improvement between the second two time points (z=–2.388, p=0.016). Patients reported that the improvement in their mood was mainly caused by the reduction in their tics.
At final follow-up, there was significant improvement in mean scores on the Gilles de la Tourette Syndrome–Quality of Life Scale, from 39.09 to 79.09 (z=–2.94, p=0.001), and on the GAF, from 47.27 to 74.55 (z=–2.82, p=0.002), relative to baseline.
No patient was completely tic free following DBS, but only two patients required ongoing medication specifically targeting tics. Another two patients required other psychotropic medication to target depression or anxiety. One patient continued to receive botulinum toxin. Overall, there was a considerable reduction in the requirement for pharmacotherapy following DBS. Patient 2, who had a particularly severe case of Tourette's syndrome, responded well to DBS, although there was only a marginal improvement in her total score on the Yale Global Tic Severity Scale following DBS (a 12% lower score). She improved substantially in her overall functioning and was able to live much more independently and engage in further study, and her family relationships improved substantially. It is likely that the marginal improvement in her Yale Global Tic Severity Scale total score was a reflection of the fact that her baseline scores were at the ceiling.
No intraoperative or immediate postoperative complications were observed in any patient. Patients typically reported a reduction in anxiety by the first postoperative day and a reduction in tic severity by the second or third. Postoperative cerebral imaging of all patients confirmed that the electrodes were in the anteromedial globus pallidus interna in all cases (
Figure 1C).
Initial stimulation parameters were 1 V, pulse width 60 msec, and frequency 130 Hz. Stimulation parameters were adjusted at follow-up visits depending on residual tic severity and the presence of any side effects. At final assessment, amplitude parameters were between 3 V and 5 V, pulse width was between 60 and 120 msec, and frequency was between 100 Hz and 160 Hz.
Adverse Effects
In general, DBS was well tolerated. The only serious adverse effect reported was increased tic severity in patient 11. There were no intraoperative adverse outcomes and no reports of suicidal thoughts or attempted suicides. Complications resulting from hardware malfunction occurred in three patients. One patient had a car accident 3 months after surgery that resulted in lead breakage that required repair. Another patient inadvertently caused lead damage by hitting her chest as part of her self-injurious tic. A third patient's lead broke for no apparent cause. Each of these patients reported worsening tic severity during the time they were without DBS stimulation and an improvement once the leads were repaired. Another patient reported accidently turning off the device and difficulty with charging the device, which left her without stimulation for some periods of time. One patient reported a persistent but fluctuating increase in anxiety with panic attacks, and a second patient reported transient anxiety following an increase in stimulation dose. This anxiety was managed by adjusting the stimulation parameters very gradually. One patient developed an infection around the neck leads 3 months after surgery and required bilateral lead replacement. Another patient reported neck discomfort that he attributed to the leads and one occasion of “electric shock” sensation while touching an electrical appliance. This was the same patient whose lead broke for no apparent cause.
Discussion
This study provides the largest reported case series of patients who underwent DBS in the anteromedial globus pallidus interna for treatment-refractory Tourette's syndrome. All but one of the 11 patients reported a significant improvement in tic severity and quality of life following DBS, with an overall 51% reduction in total tic severity relative to baseline. In six patients (54.5%), the tic severity total score on the Yale Global Tic Severity Scale was reduced by more than 50%. There was an associated improvement in measures of depression and overall quality of life as well as level of functioning. Seven patients who had obsessive-compulsive symptoms reported an overall improvement in symptoms, with a 59% reduction in the mean Y-BOCS score.
These results suggest that the globus pallidus interna is a safe and effective target for severe treatment-refractory Tourette's syndrome as well as its common comorbidities such as OCD and major depression. These results are exciting because they provide further evidence that DBS is an effective treatment option for a subgroup of patients for whom no other treatment option is available. Furthermore, globus pallidus interna DBS appears to have an impact not only on tic severity, obsessive-compulsive symptoms, and depression but also on the overall quality of life and general level of functioning of patients with treatment-refractory Tourette's syndrome. An additional effect is the reduced need for ongoing pharmacotherapy and thus a reduction in the risks of long-term treatment with dopamine antagonists and other anti-tic medications. Although there is a larger up-front cost to the patient, the long-term costs may be significantly reduced compared with long-term pharmacotherapy.
Currently, there is no consensus on the most appropriate DBS target site. This is possibly because of the relatively small number of patients who have undergone DBS for Tourette's syndrome globally and because no fewer than nine anatomical sites have been targeted with varying results. We chose to stimulate the anteromedial globus pallidus interna in our patients because it is the main output regulator of the basal ganglia and appears to play a role in controlling motor function and behavior. In addition, there is preliminary evidence that globus pallidus interna DBS has yielded more favorable results than stimulation of other targets. Large randomized double-blind studies are yet to be done. To date, there have been only two small studies with blinded analysis, both of which yielded favorable results. One factor described by Hariz and Robertson (
2) that may complicate the analysis of a target site is that a higher stimulation level may cause the current to spread to surrounding structures, making evaluation of the precise target site difficult. This, together with the phenotypic variability of Tourette's syndrome and its various expressions of comorbidities, makes it even more difficult to assess precisely which circuitries are modulated.
It is interesting that the patients who responded favorably to DBS reported an improvement in symptoms within 1 to 3 days, which suggests that DBS has a relatively rapid onset of action compared with other treatment modalities such as medication or behavioral therapy. This was further evidenced by the fact that patients who had a temporary absence of stimulation reported a recurrence of tics followed by an improvement immediately upon reestablishment of stimulation, linking stimulation with tic improvement as distinct from the implantation phenomenon that has been described following surgery (
13). These results were sustained over the course of follow-up (up to 30 months). Although minor adjustments to stimulation parameters were required over the course of the study, the sustained improvement suggests that the effect of stimulation persisted without any effect of fatigue or tolerance.
Paired comparison between the two post-DBS follow-up assessments was significant for the HAM-D, suggesting an ongoing improvement with time. It is unclear whether the improvement in depression is a direct consequence of reduction in tic severity or whether the DBS has an independent and direct antidepressant effect. DBS has been shown to be an effective treatment for refractory depression, with response rates of about 60% (
14). However, anatomical sites targeted for depression are different from those for Tourette's syndrome and include the subcallosal cingulate gyrus, ventral capsule, and nucleus accumbens. In our study, patient reports suggested that the reduction in depressive symptoms was largely a result of reduced tic severity, but more research is needed to assess the globus pallidus interna as a potential DBS target for the independent treatment of depression or for improved functional outcomes.
The 10 patients who responded favorably to DBS reported their overall impression of DBS as highly favorable for their Tourette's symptoms, overall quality of life, and functioning. All reported an improvement in their self-confidence, a reduction in the stress their illness created in their interpersonal relationships, and an improvement in general sense of well-being.
One patient reported increased motor tics as well as other somatic symptoms with DBS. He subsequently turned the device off, and his tic severity promptly returned to baseline. Lack of efficacy or worsening of symptoms may occur as a result of poor lead wire placement in the brain, wire malfunction, inadequate stimulation parameters, undermedication, incorrect diagnosis, and inappropriate patient expectations (
15). While the electrodes were placed accurately in the target sites, the poor outcome in this patient was most likely a combined consequence of inadequate stimulation parameters and inappropriate patient expectations. He was also highly anxious, and this may account for the experience of nonspecific somatic complaints.
There were no intraoperative or immediate postoperative complications, and DBS was well tolerated. The most common adverse effects reported were hardware malfunction and anxiety and panic symptoms. Behavioral changes such as depression, anxiety, hypomania, suicidality, apathy, and aggression have previously been associated with DBS (
16). This emphasizes the importance of closely monitoring all patients after this procedure. In studies to date, other reported adverse effects have included feelings of decreased energy, changes in libido (
17), and one case of vertical gaze palsy (
18). There are few serious adverse effects from either surgery or stimulation. The reported incidence of neurological adverse events such as stroke has been as high as 5%, but these data largely come from patients receiving DBS for Parkinson's disease, and the lower incidence of neurological morbidity reported in the Tourette's syndrome patients may reflect the younger age at the time of surgery. Nonetheless, the potential for serious adverse consequences is not absent in Tourette's syndrome, and careful patient selection in an experienced functional neurosurgical program is paramount. Tourette's syndrome patients have been reported by one group to be more likely to develop infective complications at the implanted hardware site (
19).
There are a number of limitations to this study. First, the open-label study design is prone to interviewer and patient bias, even though the assessments were performed independently of the surgical team. However, the consistency of the results from both objective and self-report scales, the persistence of effects, the chronicity of the symptoms before DBS, and the recurrence of symptoms when DBS was inadvertently interrupted in a few cases, argue against this being a placebo effect. Randomized, double-blind, and sham-controlled studies are lacking in this area and should be conducted to further substantiate the evidence for this treatment. Second, the sample size is small even if this is the largest case series of globus pallidus interna DBS for treating Tourette's syndrome from one center. The small sample size does not permit the examination of predictors of good response or a detailed examination of comorbidities. Third, further follow-up is needed to evaluate longer-term outcomes and adverse effects. In addition, head-to-head studies comparing DBS with different targets would help establish the best anatomical sites for stimulation.