Increase in Work Productivity of Depressed Individuals With Improvement in Depressive Symptom Severity
Abstract
Objective
Method
Results
Conclusions
Method
Study Description
Study Participants
Assessment of Sociodemographic and Clinical Characteristics
Work Productivity and Health-Related Quality of Life Measures
Statistical Analysis
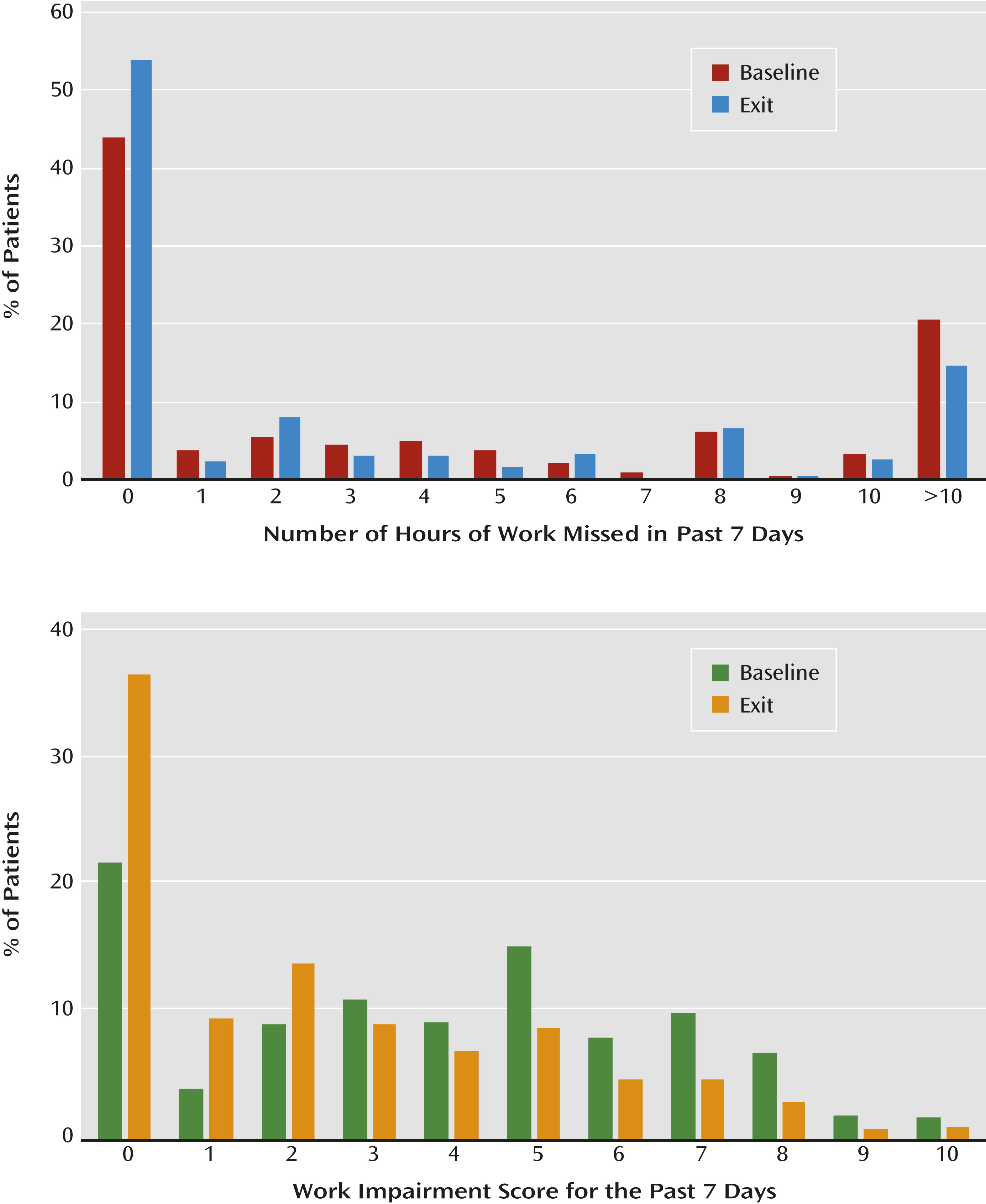
Results
| Number of Hours Missed | Impaired Productivity | ||||
|---|---|---|---|---|---|
| Factor | β | SE | p | Odds Ratio | p |
| Race (reference, white) | 0.003 | 0.03 | |||
| Black | 0.29 | 0.10 | 0.68 | ||
| Other | –0.07 | 0.14 | 1.01 | ||
| Insurance (reference, private) | 0.007 | ||||
| Public | 0.08 | 0.15 | |||
| None | –0.22 | 0.07 | |||
| Education (reference, <college) | 0.001 | ||||
| <High school | 0.06 | ||||
| ≥College | 1.26 | ||||
| Attempted suicide | 0.25 | 0.09 | 0.006 | 0.73 | 0.01 |
| Anxious features | 0.26 | 0.07 | <0.001 | 1.45 | <0.001 |
| Age at onset (reference, ≤18 years) | 0.24 | 0.07 | <0.001 | ||
| Baseline QIDS-IVR score (units=5) | 0.07 | 0.01 | <0.001 | 1.80 | <0.001 |
| ≥50% Improvement in Hours Missed | ≥50% Improvement in Impaired Productivity | |||
|---|---|---|---|---|
| Factor | Odds Ratio | p | Odds Ratio | p |
| Education (reference, <college) | 0.006 | |||
| <High school | 5.32 | |||
| ≥College | 1.30 | |||
| Melancholic features | 0.47 | 0.002 | ||
| Atypical features | 0.56 | 0.02 | ||
| Recurrent depression | 0.51 | 0.005 | ||
| Change in QIDS-IVR score (units=5) | 1.91 | <0.001 | 3.16 | <0.001 |
| Age group (years) | 0.03 | |||
| 26–35 | 1.55 | |||
| 36–50 | 0.77 | |||
| 51–65 | 1.25 | |||
| 66–75 | 8.95 | |||
| Baseline | Exit | |||||
|---|---|---|---|---|---|---|
| Response Status and Measure | N | Mean | SD | N | Mean | SD |
| Nonresponse | 894 | 435 | ||||
| Hours missed | 8.1 | 12.3 | 6.3 | 10.7 | ||
| Impairment score | 4.1 | 2.9 | 3.6 | 2.7 | ||
| Response (nonremission) | 236 | 151 | ||||
| Hours missed | 6.8 | 10.1 | 3.3 | 6.7 | ||
| Impairment score | 4.3 | 2.7 | 2.2 | 2.3 | ||
| Remission | 794 | 511 | ||||
| Hours missed | 5.7 | 10.2 | 2.0 | 6.5 | ||
| Impairment score | 3.4 | 2.7 | 1.0 | 1.7 | ||
| Response Status and Measure | Switch | Augmentation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sertraline | Bupropion | Venlafaxine | Citalopram Plus Bupropion | Citalopram Plus Buspirone | |||||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Nonresponse | |||||||||||||||
| Hours Missed | 16 | –2.1 | 17.3 | 24 | –3.6 | 9.6 | 15 | –8.1 | 15.2 | 20 | –4.6 | 11.9 | 18 | 2.2 | 14.2 |
| Impairment | 21 | –1.1 | 2.7 | 32 | –0.9 | 2.2 | 23 | –0.5 | 3.2 | 22 | –1.5 | 2.8 | 29 | –1.3 | 3.5 |
| Partial Response | |||||||||||||||
| Hours Missed | 1 | –28 | 1 | –25 | 5 | –4.8 | 7.1 | 1 | 10 | 3 | –3.0 | 2.6 | |||
| Impairment | 0 | 1 | –2 | 8 | –3.1 | 3.2 | 1 | –1 | 5 | –2.4 | 1.5 | ||||
| Remission | |||||||||||||||
| Hours Missed | 16 | –3.5 | 15.7 | 7 | –4.7 | 4.2 | 3 | –15.3 | 21.5 | 17 | –5.7 | 14.2 | 18 | –5.4 | 9.7 |
| Impairment | 21 | –1.6 | 2.4 | 15 | –1.4 | 2.3 | 9 | –2.4 | 2.2 | 27 | –2.7 | 2.9 | 27 | –2.1 | 2.1 |
Discussion
Conclusions
Acknowledgments
Footnote
Supplementary Material
- View/Download
- 138.21 KB
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

