Developmental models of autism spectrum disorders (ASDs) suggest that atypical or biased visual attention patterns in the first year of life could directly contribute to the emergence of the characteristic social-cognitive deficits (
1). Indeed, many of the early behavioral markers associated with a later emerging ASD—orienting to name, responding to bids for joint attention, spontaneous gaze to faces, and making eye contact (
2–
4)—implicate behaviors associated with flexibly allocating attentional resources to salient or biologically relevant information in the environment.
Selective attention refers to the process by which information is channeled, filtered, or enhanced for further processing in higher-order neural systems. It has long been recognized that selective attention shapes the development of adaptive cognitive function (
5). Although the neural circuitry of selective attention has been comprehensively delineated in adults (
6–
10) and selective attention’s role in social cognition continues to be elucidated (
10,
11), the precise circuitry that underlies selective visual attention during infancy is unknown. Characterizing the neural correlates of visual attention during infancy may inform our understanding of cortical specialization, as experience with specific categories of information may sculpt neural circuits and enhance processing efficiency (
12).
The specialized perception, processing, and evaluation of social information readily observable in typically developing individuals are a source of impairment in individuals with ASDs. Navigating the subtle complexities of social dynamics often requires rapid, flexible, and efficient information processing. But before the actual processing of social information, flexibly orienting gaze and visual attention to the most salient or biologically relevant information in the environment is essential. We reasoned that a deficit in visual orienting during infancy could constrain typical developmental processes leading to specialization with social information, thereby contributing to the emergence of social-communication deficits related to a later diagnosis of ASD. In one study (
13), 9- to 10-month-old infants at high familial risk for developing autism (N=16) were reported to have longer visual orienting latencies compared with typically developing low-risk infants. Another study (
2) reported that visual orienting latencies measured in a high-risk cohort at 12 months (N=27) correlated with autism symptom severity at 24 months. While these studies informed our hypotheses in this study, critical questions remain as to whether compromised visual orienting behavior is specific to infants who later express an ASD, and if so, how early this behavioral pattern can be detected.
We employed a well-validated visually guided saccade task (the gap/overlap paradigm) to examine saccade latencies in two conditions that represent visual orienting (overlap condition) and oculomotor efficiency (gap condition), respectively. We also used diffusion tensor imaging (DTI) to delineate specific white matter fiber tracts hypothesized to be associated with these behaviors. A recent DTI study reported a unique association between orienting and the splenium of the corpus callosum in adults (
9). The splenium is the most posterior sector of the corpus callosum and projects to striate and extrastriate visual areas and to portions of the posterior parietal cortex, including Brodmann’s area 7 (
14), a cortical target implicated in sensorimotor programming (
15). This tract may function in part as transitional connectivity between extrastriate visual areas and the dorsal frontoparietal and ventral frontoparietal orienting networks identified in adults (
10).
Saccadic eye movements that represent oculomotor efficiency, as operationalized in this context, result from temporary inhibition of fixation neurons in the rostral pole of the superior colliculus and disinhibition of the saccade-generating circuit, mediated in part by the reticular formation in the brainstem (
16). We therefore hypothesized that individual differences in the organization of white matter fiber bundles projecting from the brainstem to cortical targets (namely, corticospinal tracts) would be associated with individual differences in oculomotor efficiency. Finally, we examined the genu of the corpus callosum as a control tract. The genu projects to frontal association areas associated with voluntary, goal-directed attentional operations (
9).
Our goal in this study was to determine whether patterns of oculomotor efficiency and visual orienting in 7-month-olds who are later classified as expressing ASD symptoms are different from those of other infants of similar age. We also examined the neural correlates of task-based behavioral performance and whether the patterns of brain-behavior associations differed between groups.
Method
Sample
This study was conducted in the context of an ongoing Autism Center of Excellence Network study prospectively investigating longitudinal brain and behavioral trajectories in high-familial-risk infant siblings of children with ASD and low-risk comparison infants. Infants were considered at high familial risk if they had a biological sibling diagnosed as having an ASD. All infants were enrolled at approximately 6 months of age and underwent a comprehensive behavioral assessment and brain imaging protocol around 6, 12, and 24 months of age (the actual chronological age of children at the time of the visit lagged slightly behind the target visit stages). The data used to investigate the hypotheses in this study include behavioral, imaging, and eye-tracking data from the initial 6-month visit stage and clinical assessment data from the 24-month visit stage. The children were assessed at the University of North Carolina at Chapel Hill and the Children’s Hospital of Philadelphia. The research protocol was approved by the institutional review boards at both sites, and parents provided written informed consent after receiving a detailed description of the study. Exclusion criteria for both high-familial-risk and low-familial-risk (hereafter high-risk and low-risk) children included the following: history or physical signs of known genetic conditions or syndromes; significant medical or neurological conditions affecting growth, development, or cognition or sensory impairments such as significant vision or hearing loss; birth weight <2000 g or gestational age <36 weeks, a history of significant perinatal adversity, or in utero exposure to neurotoxins (including alcohol, illicit drugs, and selected prescription medications); a contraindication for MRI; a predominant home language other than English; having been adopted; and a family history of a first-degree relative with an intellectual disability (only for the low-risk group), psychosis, schizophrenia, or bipolar disorder. Low-risk infants were excluded if they had a family history of a first- or second-degree relative with autism or if the low-risk proband (older sibling) showed symptoms of ASD on the Social Communication Questionnaire (
17). Clinical diagnosis was corroborated in the proband of high-risk infants with the Autism Diagnostic Interview–Revised (
18).
Infants were included in this study if they participated in the eye-tracking protocol during the 6-month visit and had an outcome classification at the 24-month visit. A total of 113 infants were eligible for the study according to these criteria. Fourteen infants did not complete a sufficient number of trials in the eye-tracking task (low-risk group, N=8; high-risk group, N=6) and were excluded from subsequent analyses. These infants did not differ in age or developmental level from those who were included in subsequent analyses. Two infants were enrolled as low-risk but subsequently exceeded the Autism Diagnostic Observation Schedule threshold for an ASD and were excluded from the analyses. The final sample included 97 infants.
Table 1 summarizes the sample’s characteristics.
Clinical Assessment
Primary cognitive assessments during the 6-month visit stage included the Mullen Scales of Early Learning (
19). ASD classification was made during the 24-month visit with the Autism Diagnostic Observation Scale (ADOS) (
20). The ADOS was used to maximize reliable classification of ASD symptoms and was administered and scored by experienced, research-reliable clinicians. A total of 97 infants completed the experimental task and were categorized into three groups based on risk status and ASD classification on the ADOS: low-risk infants (N=41), high-risk infants who did not meet ASD criteria on the ADOS (high-risk-negative group, N=40), and high-risk infants who met ADOS criteria for an ASD (high-risk-ASD group, N=16). Seven infants in the low-risk group, four in the high-risk-negative group, and two in the high-risk-ASD group contributed valid eye-tracking data but did not contribute valid imaging data, leaving 84 infants with valid imaging data and eye-tracking data.
Eye-Tracking Procedure
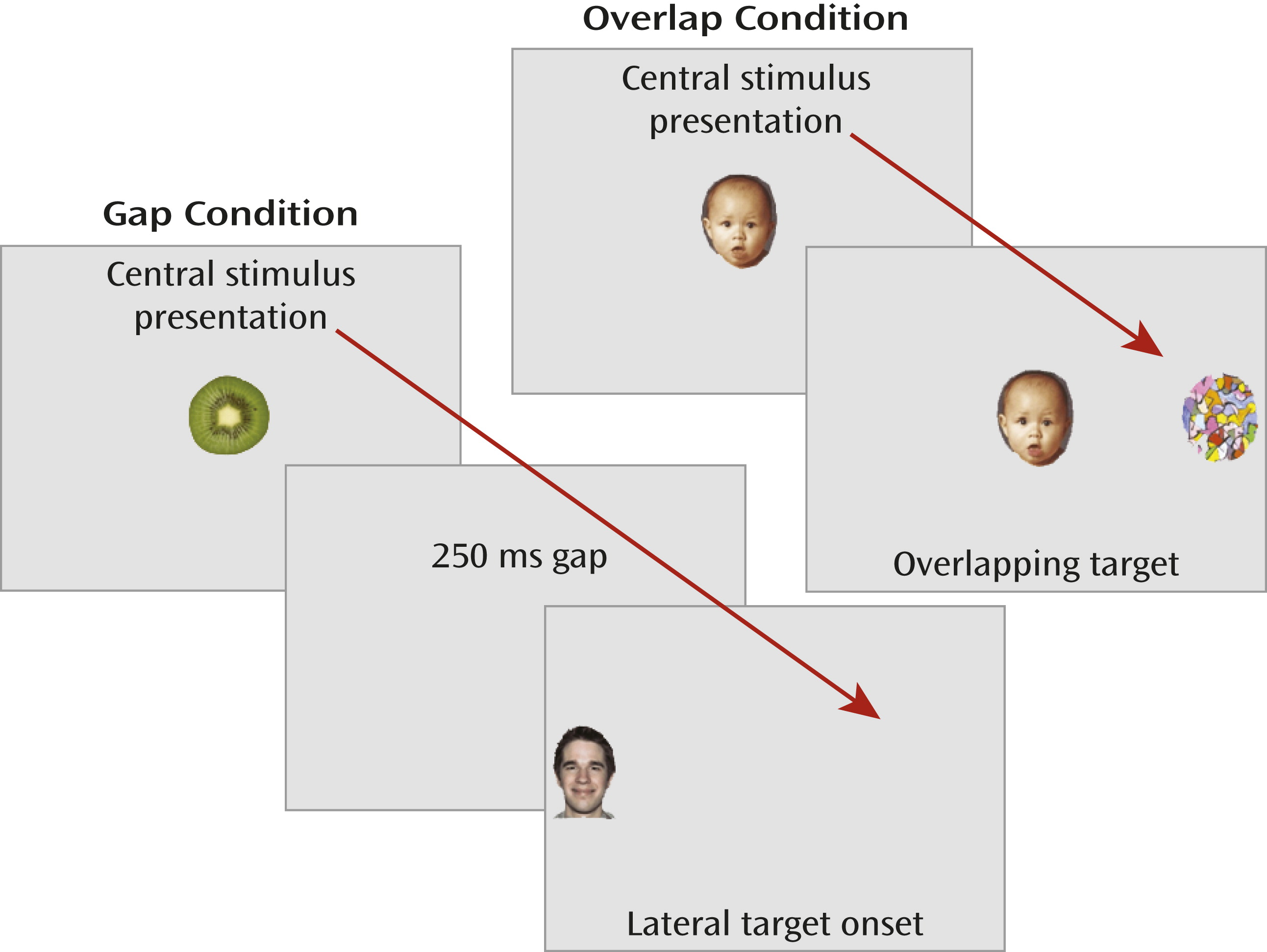
We measured overt stimulus-driven orienting during visually guided saccade trials in which the timing of the fixation offset and target-stimulus onset was manipulated to examine oculomotor efficiency and visual orienting (
Figure 1). Eye movements were recorded with corneal-reflection binocular eye-tracking equipment (Tobii models 1750 and x120, recordings sampled at 50 and 60 Hz, respectively; Tobii Technology, Danderyd, Sweden). The primary dependent measure was latency to initiate a saccade away from the center image (or center of the display in the gap condition) in the correct direction of the target. Average saccadic latency in the gap condition represents raw oculomotor efficiency. The offset of the fixation stimulus primes the oculomotor system and results in the temporary inhibition of fixation neurons in the rostral pole of the superior colliculus and disinhibition of the saccade-generating circuit, mediated in part by the reticular formation in the brainstem (
16). Individual differences in average gap latency stem in part from the relative computational efficiency of these circuits; hence, we refer to “oculomotor efficiency” when describing saccadic latencies in the gap condition. Average saccadic latency in the overlap condition encapsulates a combination of oculomotor responding and attentional orienting (disengaging and shifting attention). Overlap latencies are consistently longer than gap latencies, likely resulting from 1) additional processing demands supported by the lateral intraparietal area (
16,
21,
22) and 2) the absence of the fixation offset priming effects. We refer to saccadic latencies in the overlap condition as “visual orienting,” as this concept subsumes both attentional and oculomotor components.
This study was primarily concerned with domain-general response patterns, as there is evidence to suggest that 6-month-old infants who later develop ASD exhibit patterns of “gaze to faces” and “gaze to objects” similar to those of 6-month-olds who do not develop ASD (
4). Therefore, we included a variety of complex stimuli (parametrically varied among central and peripheral locations) that included 10 distinct faces and 10 distinct objects. Trials were counterbalanced with no direction (i.e., left or right), condition (i.e., gap or overlap), or central or peripheral stimulus type, respectively (i.e., faces or objects) occurring on more than three consecutive trials. More information about the procedure is available in the
data supplement that accompanies the online edition of this article.
Imaging Protocol
Diffusion-weighted images were acquired during natural sleep on a Siemens 3-T TIM Trio scanner (Siemens, Munich) equipped with a 12-channel head coil using the following parameters: field of view=190 mm, 75 transversal slices, slice thickness=2 mm isotropic, 2×2×2 mm
3 voxel resolution, TR=12800 ms, TE=102 ms, b values of 0 to 1000 s/mm
2, 25 gradient directions, and 4- to 5-minute scan time. Image data were screened for motion and common artifacts using the DTIprep program (
www.nitrc.org/projects/dtiprep), and expert raters manually removed scans with residual artifacts.
Computational Anatomy Mapping
Image registration proceeded in two steps (
23). First, linear affine registration of baseline images was applied to the structurally weighted T
2 atlas using B-spline registration and normalized mutual information (
24). Second, a large deformation diffeomorphic metric mapping transformation was applied for unbiased, deformable atlas building (
25). The procedure related each individual data set to the study-specific atlas template via a nonlinear, invertible transformation, providing a precise match between the reference atlas and individual image data. Tensor maps were calculated from each data set using weighted least squares estimation (
26) and were transformed into the atlas space with tensor reorientation by a finite strain approach (
27). Diffusion tensor images were transformed and averaged using the Riemannian and log-Euclidean frameworks (
28), resulting in the final three-dimensional average tensor atlas.
Segmentation and Parameterization of Fiber Tracts
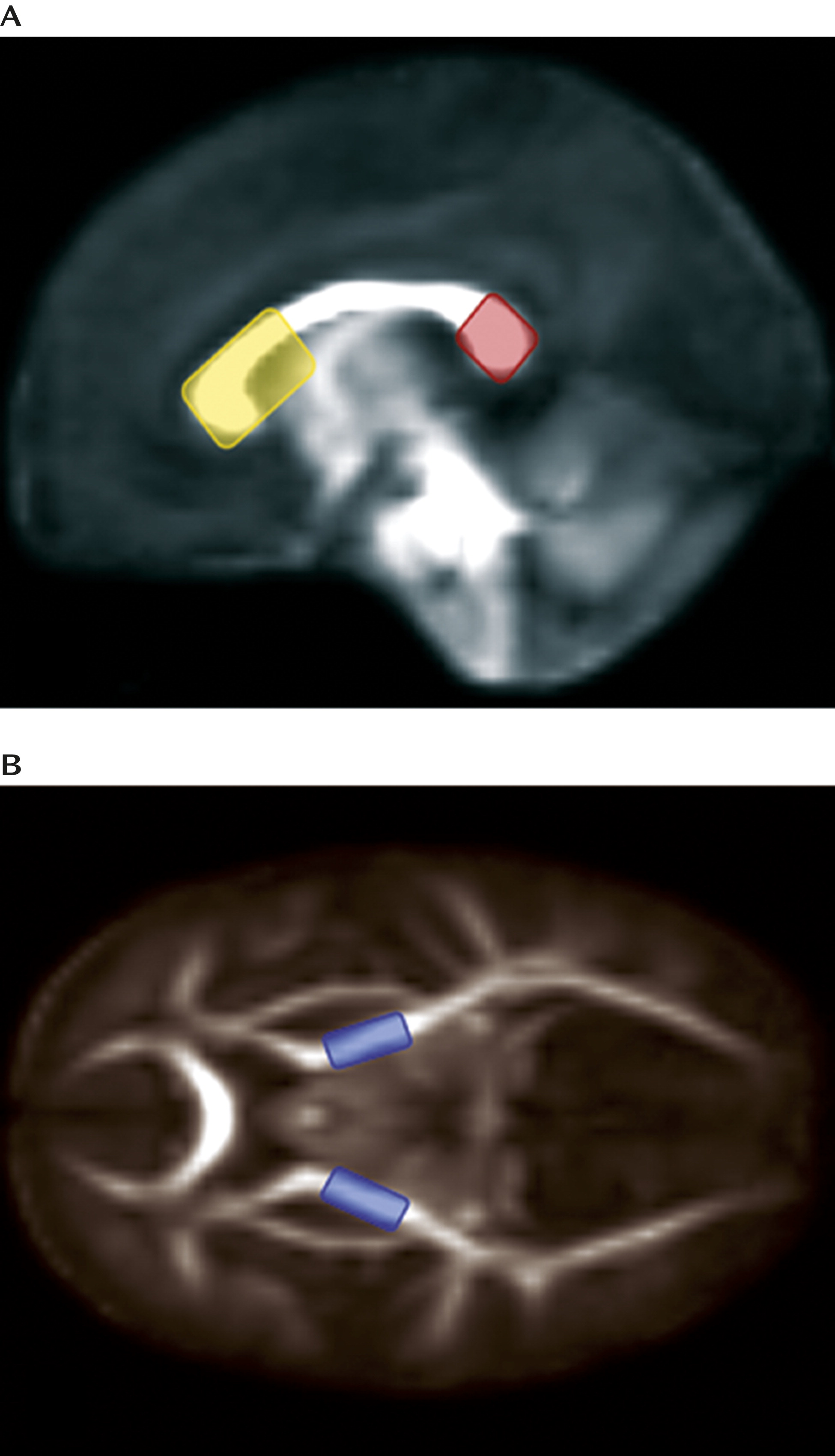
Deterministic tractography was performed by manually drawing seed label maps for the splenium and genu of the corpus callosum and the left and right corticospinal tracts (broadly defined as corticospinal pathways passing through the posterior limb of the internal capsule) based on methods described previously (
29) using the 3DSlicer program (
www.slicer.org) (
Figure 2). Seed spacing was set at 1 mm and proceeded bidirectionally with a linear measure stopping value of 0.1. Extracted fiber tracts were processed for spurious or incomplete streamlines using a software program developed in-house (FiberViewer;
www.ia.unc.edu/dev/). Fiber tracts were converted to binary regions of interest and mapped to individual data sets to generate scalar diffusion measures (i.e., radial diffusivity, axial diffusivity, and fractional anisotropy) along each fiber bundle, from which mean values were derived. We initially chose to focus on radial diffusivity because there have been reports linking this metric to myelination (
30), and the relative myelin content is thought to support rapid and efficient signal transmission (
31) and therefore efficient information processing. However, there is evidence to suggest that the density and diameter of axons in a given fiber tract, in addition to myelin content, affect the diffusion of water molecules perpendicular to the primary eigenvector of a fiber tract (
32). Radial diffusivity was calculated in the conventional manner as the mean of the second and third eigenvalues ([λ
2+λ
3]/2). To supplement analyses of radial diffusivity reported below, we also examined axial diffusivity and the composite index, fractional anisotropy. The results from these additional analyses are reported in the online
data supplement.
Results
The sex ratio did not differ significantly across the three groups of children, and the groups did not differ statistically in age at the initial 6-month visit, age at the 24-month clinical visit, nonverbal developmental quotient as measured by the Mullen Scales of Early Learning at the 6-month visit, or standardized score on the visual reception subscale of the Mullen Scales of Early Learning at the 6-month visit.
A multivariate analysis of variance that included gap latency and overlap latency as dependent variables and risk status (low-risk, high-risk-negative, and high-risk-ASD) as the independent variable was statistically significant (Wilks’s lambda=0.879; F=3.097, df=4, 188, p=0.017; η
p2=0.062). The statistical model also revealed a significant main effect of risk status on both gap latencies (F=3.14, df=2, 94, p=0.048; η
p2=0.063) and overlap latencies (F=3.42, df=2, 94, p=0.037; η
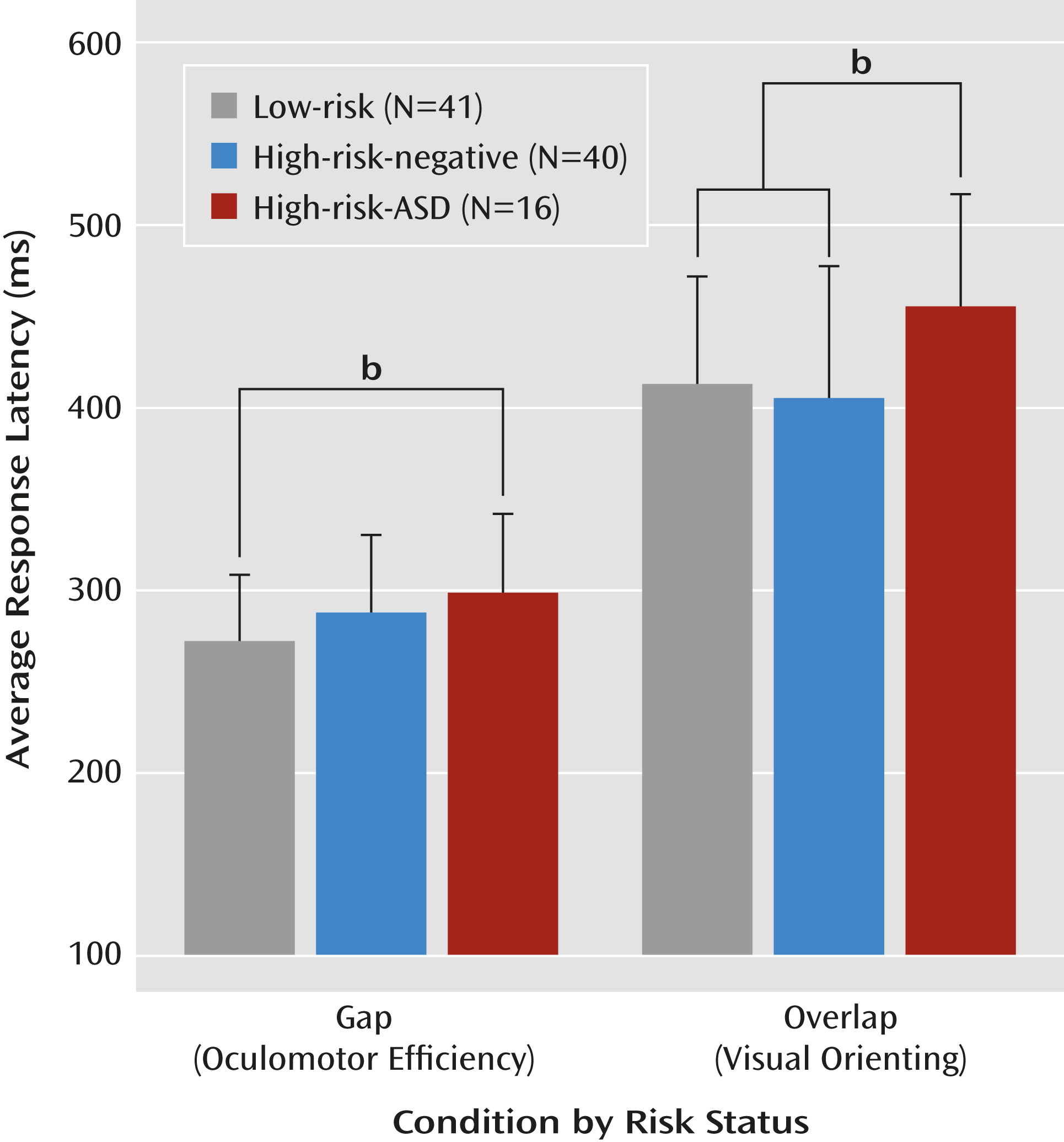
p2=0.068). Planned post hoc comparisons revealed that the high-risk-ASD group showed significantly longer gap latencies than the low-risk group (d=0.71, p=0.025) and that the high-risk-negative group did not differ significantly from the low-risk or high-risk-ASD groups. Considering the overlap condition, the high-risk-ASD group showed significantly longer latencies than both the high-risk-negative group (d=0.73, p=0.012) and the low-risk group (d=0.71, p=0.032). The low-risk and high-risk-negative groups showed statistically equivalent overlap latencies.
Table 2 and
Figure 3 summarize performance on the gap/overlap task.
Next, we tested the specificity of the association between average saccadic latencies in the gap condition and white matter fiber tracts that transmit information to and from the brainstem (i.e., the corticospinal tract) and the association between average saccadic latencies in the overlap condition and the splenium of the corpus callosum. To accomplish this, we also examined the association between the saccadic latencies and the genu of the corpus callosum to confirm the specificity of any observed association with the splenium or the corticospinal tracts. We report results using radial diffusivity and provide additional data on fractional anisotropy and axial diffusivity when relevant. The analytic strategy (see also reference
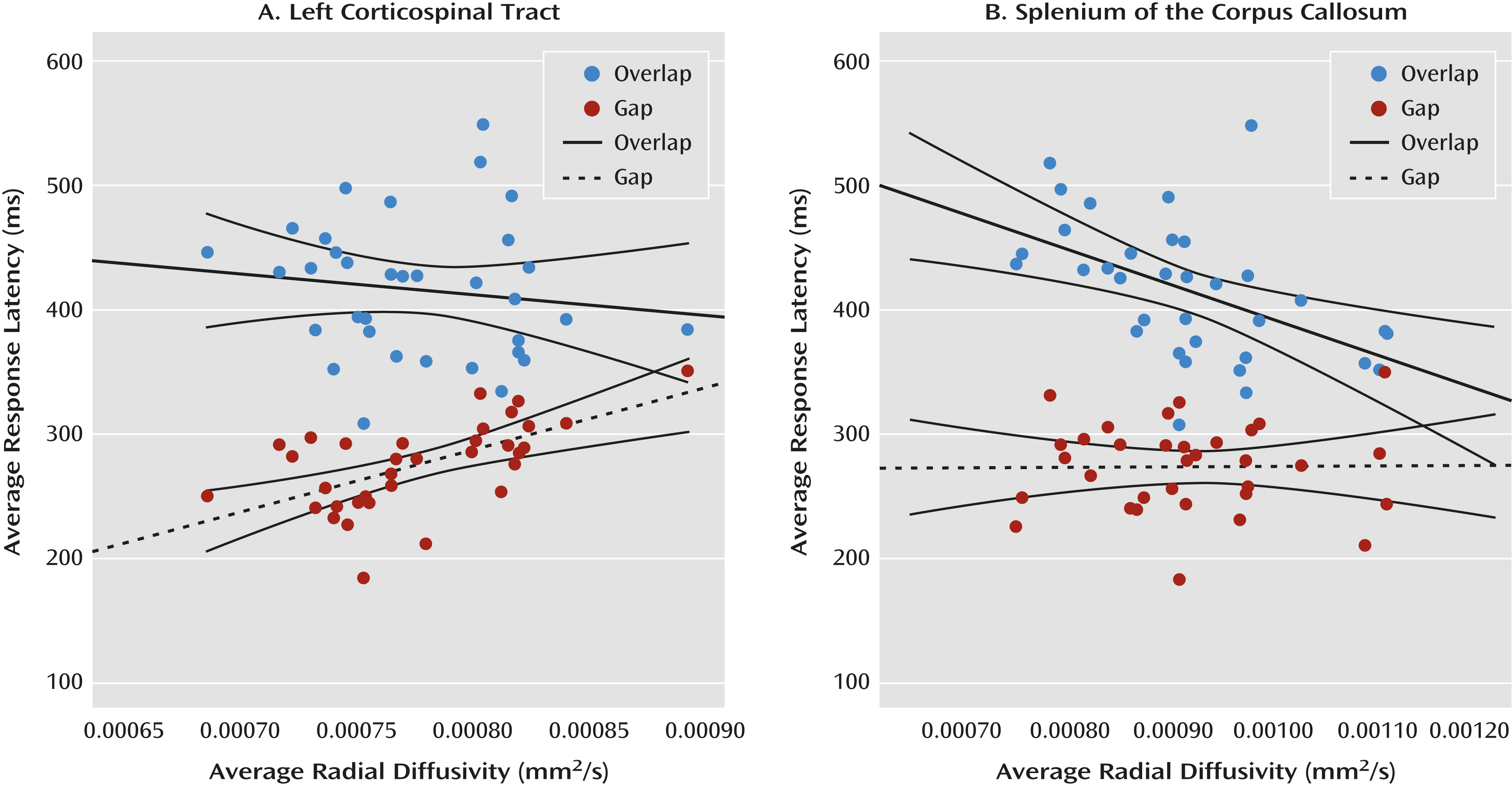
9) began with an examination of the zero-order correlation matrix (see graphical representation in
Figure 4) and followed with hierarchical multiple regression analyses. The scalar index of the fiber tract of interest was entered into the model as the dependent variable, age and the behavioral response unrelated to the fiber tract were entered as step 1 predictor variables, and the behavioral response hypothetically related to the fiber tract was entered as a step 2 predictor variable.
These analyses verified that performance in the two conditions of the gap/overlap procedure was related to dissociable white matter fiber tracts in low-risk infants (N=34; mean age, 31.0 months [SD=3.5]). Average latency in the gap condition accounted for a significant portion of variance in radial diffusivity in the left corticospinal tract (∆R
2=0.486, p<0.001) beyond the contribution of age and overlap latency. Average latency in the overlap condition accounted for a significant portion of variance in radial diffusivity in the splenium beyond the contribution of age and gap latency (∆R
2=0.297, p=0.001). Gap latencies did not account for a significant portion of variance in the splenium, and overlap latencies did not account for a significant portion of variance in the left corticospinal tract. There was no association between latencies in the gap/overlap procedure and our control tract, the genu of the corpus callosum (see Figure S2 in the online
data supplement). All of these patterns remained when we examined fractional anisotropy and axial diffusivity (see the
data supplement), except that gap latencies did not account for a significant portion of the variance in fractional anisotropy in the left corticospinal tract (∆R
2=0.097, p=0.076) beyond the effect of age and overlap latency. However, the trend toward statistical significance is consistent with the results from radial and axial diffusivity.
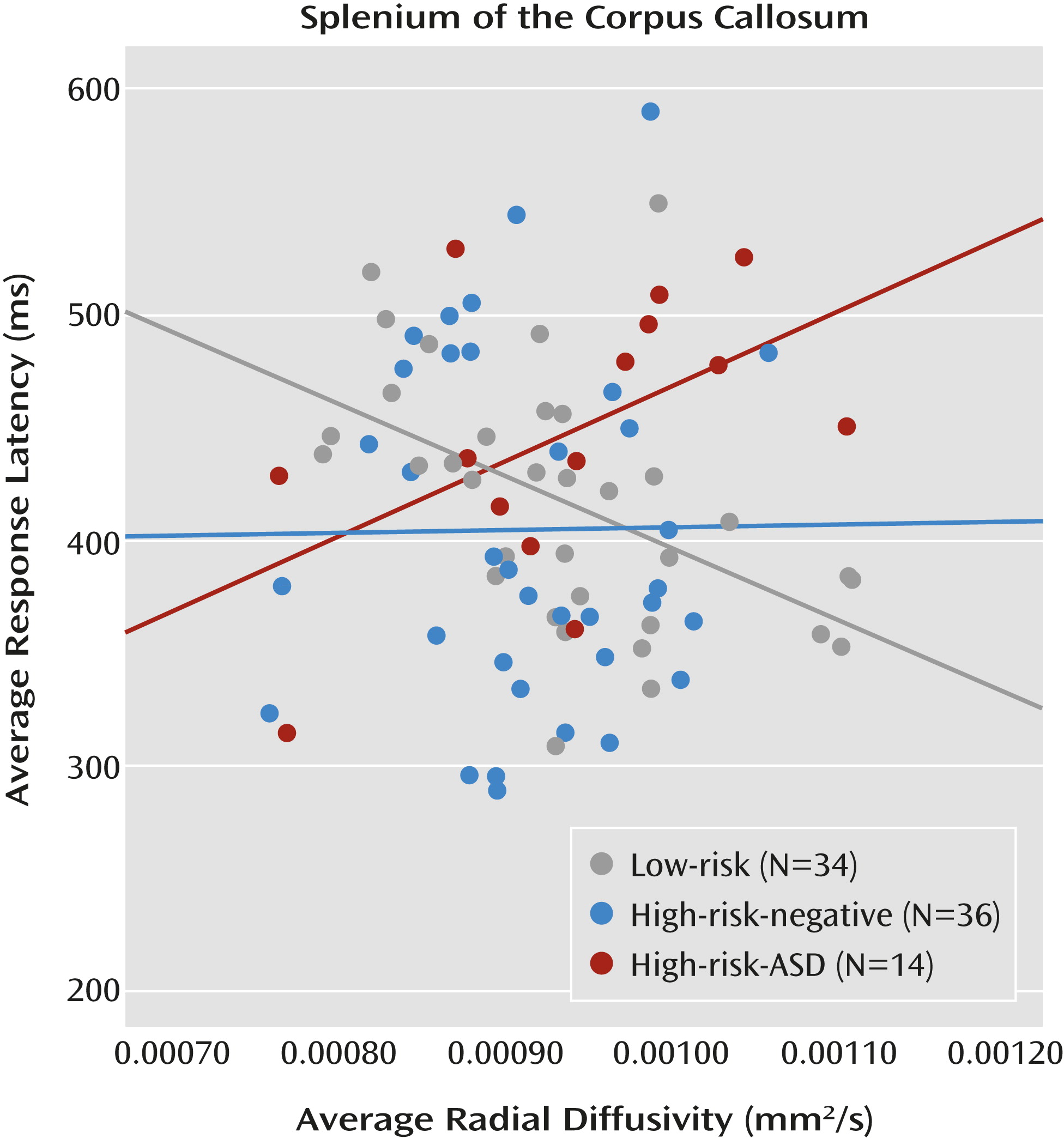
Considering the pattern of results in the overlap condition (high-risk-ASD > high-risk-negative = low-risk), alongside the finding that overlap latencies (visual orienting) are associated with the splenium in low-risk infants, directly informed the final question: Do the functional properties of the splenium explain the increased overlap latencies observed in infants who later express ASD symptoms? We employed a general linear model that included overlap latencies as the dependent variable and group, radial diffusivity in the splenium, and a group-by-splenium interaction term as independent variables. Group status moderated the association between white matter microstructure in the splenium and overlap latencies. The overall model was significant (F=2.82, df=5, 78, p=0.021). Additionally, the results revealed a main effect of group (F=4.16, df=2, 78, p=0.019; η
p2=0.096) as well as a significant group-by-splenium interaction (F=4.53, df=2, 78, p=0.014; η
p2=0.104). Average radial diffusivity in the splenium did not differ between groups. The significant interaction indicates that the association between brain and behavior varies by group. The simple slope for the low-risk group significantly differed from the simple slope for the high-risk-ASD group (t=−2.95, p=0.004). The simple slope for the high-risk-negative group did not differ significantly from the low-risk group or the high-risk-ASD group (
Figure 5). This pattern of results, and specifically the significant difference in simple slopes between the low-risk group and the high-risk-ASD group, is corroborated by analyses conducted with axial diffusivity (see the online
data supplement). For additional results regarding the moderating effect of group status on the association between gap latencies and the left corticospinal tract, see Figures S3 and S5 in the
data supplement.
Discussion
One underlying theme of this study was to elucidate mutually informative findings regarding both typical and atypical development processes. This is among the first studies to characterize an association between specific white matter fiber tracts and specific behavioral patterns in typically developing infants, an approach that lends itself to inferences regarding functional circuits during infancy. Our results suggest that among typically developing infants, individual differences in visual orienting are associated with individual differences in white matter microstructure of the splenium of the corpus callosum. Visual orienting latencies were not related to the microstructure of the corticospinal tracts, nor were they associated with the anterior segment of the corpus callosum, the genu. Supporting our claims of specificity, oculomotor efficiency, as represented by saccadic latencies in the gap condition, was significantly associated with white matter microstructure in the corticospinal tracts, but not associated with white matter in the splenium or the genu of the corpus callosum. This pattern is consistent with studies examining DTI-related associations with attentional operations in adults (
9), as well as behavioral evidence from a rare infant hemispherectomy patient (
33).
The internal capsule and the corticospinal tracts myelinate earlier than cortical and commissural tracts (
34), and the splenium undergoes more postnatal axonal elimination than any other sector of the corpus callosum (
35), a process that likely extends into the sixth or seventh postnatal month in human infants. Therefore, different biophysical mechanisms could affect the diffusion of water molecules in the corticospinal tracts and the splenium at different ages—namely, density in the splenium and myelination in the corticospinal tract—and thus lead to different directions of association with target behaviors. Considering the density of axons as the primary constraint on water diffusion in the splenium facilitates interpretation of the negative association with visual orienting latencies. A greater density of axons (a proportion of which will subsequently be selectively eliminated) should yield less radial diffusion and could reflect a less mature and less efficient information processing system, resulting in slower reaction times. However, this putative interpretation should be qualified by a growing literature that promotes extreme caution when making inferences about the underlying tissue structure that constrains water diffusion along and across fiber bundles (
36). Future research using targeted imaging pulse sequences (
34) has the potential to elucidate specific neuroanatomical mechanisms that underlie information processing during infancy.
Previous research has shown that visual orienting latencies are related to encoding speed in 4-month-olds (
37), which subsequently predicts cognitive outcomes in preschool-age children (
38).
Our results here reveal a disorder-specific deficit in visual orienting, such that infants who later express ASD symptoms show significantly longer latencies than both high-risk-negative and low-risk infants. By extension, these data suggest that flexible and efficient visual orienting may also be important for typical patterns of social-cognitive development.
Furthermore, these results indicate that the functional coupling between visual orienting and white matter microstructure of the splenium observed in low-risk infants differs significantly from high-risk infants who later express ASD symptoms. More research is needed to explicate the precise neural mechanisms that underlie increased visual orienting latencies observed in infants who later express ASD, but our results suggest that the functional efficiency of the splenium and the posterior heteromodal association areas may be critical.
With regard to oculomotor efficiency, or performance in the gap condition, the difference between low-risk and high-risk-ASD infants was quite large and statistically significant, which suggests a marked deficit in the saccade-generating circuit (
16,
22). However, these behavioral differences were not explained by variability in the white matter microstructure in the corticospinal tracts (see Figure S3 in the online
data supplement), suggesting that a more focused methodological approach is necessary to characterize the neural mechanism that mediates atypical oculomotor functioning in ASD. It is interesting that the high-risk-negative and high-risk-ASD groups showed statistically equivalent gap latencies. When coupled with the trend toward a significant difference between high-risk-negative and low-risk infants (d=0.41, p=0.074), this pattern of results suggests that abnormal oculomotor functioning may be a familial marker of ASD (
39).
Identifying infants at highest risk for ASD before the consolidation of syndromic features offers the possibility of implementing behavioral and other interventions during infancy that could reduce or prevent the manifestation of the full syndrome (
40). The present behavioral findings not only complement and extend recent data suggesting early functional and structural brain abnormalities in infants who go on to develop ASD (
41,
42), they also have the potential to enhance early identification of individuals with ASD and to inform targeted interventions developed for the ASD prodrome. Notably, one recent study successfully altered visual orienting performance in 11-month-olds using several gaze-contingent attention training procedures administered during five lab visits over 15 days (
43). One of the pre- and posttest procedures that tracked changes due to training was nearly identical to the gap/overlap procedure used in the present study. Additional research is needed to determine the long-term effects of modifying visual orienting patterns in infancy, specifically in relation to how training attention at specific time intervals during infancy might alter social-cognitive development.
Several limitations of this study should be mentioned. First, we used the ADOS at the 24-month visit to classify infants as either presenting ASD symptoms or not. It is currently unknown whether toddlers who show ASD symptoms at 24 months will continue to show symptoms at 3 or 4 years of age. Additionally, increasing the number of high-risk infants would afford the opportunity to examine heterogeneity within the high-risk-ASD group, as well as potential compensatory mechanisms and heterogeneity in the high-risk-negative group. Lastly, radial and axial diffusivity and fractional anisotropy represent indices of white matter microstructure but do not represent the organization of any one specific neurobiological component (e.g., myelin content). Multimodal imaging will be imperative to definitively characterize the neural mechanisms that underlie the emergence of ASD symptoms.
Acknowledgments
The authors thank the IBIS children and families for their ongoing participation in this longitudinal study. They also thank Ryan Scotton, Rachel G. Smith, Samir Das, and Penelope Kostopoulos for their efforts and Matt Mosconi, Keize Izuma, and Ralph Adolphs for their comments on the manuscript.