Attempts to identify the fundamental mechanisms of major psychiatric disorders, including their etiological underpinnings, have been remarkably unsuccessful. Defining the molecular and structural determinants of psychiatric disease is important for establishing biologically based diagnostic definitions and supporting novel targeted treatments (
1). At present, psychiatric diagnoses are based entirely on phenomenological descriptions (
2), with no assay-based biological criteria underlying diagnoses; this has resulted in syndromal categories within serious mental illness that have overlapping symptom fields (
3). This may be especially prominent among psychotic diagnoses, including schizophrenia and bipolar I disorder with psychosis. The literature documents genetic and biological overlap between schizophrenia and bipolar disorder (
4), including common risk genes (
4,
5) and overlapping family lineages with mixed psychosis diagnoses (
6–
8). To accommodate that overlap in clinical diagnosis, schizoaffective disorder is used variably in practice as an intermediate condition, with clinical manifestations of both psychosis and mood instability but with variable outcome (
9–
11). An important clinical feature shared by these diagnoses is psychosis. For the purpose of creating a cohort for genetic and molecular characterization that addresses this diagnostic overlap, psychosis provides an ideal clinical phenotype because it cuts broadly across traditional diagnostic groups (including, but not limited to, schizophrenia, schizoaffective disorder, and bipolar disorder), has a typical symptomatic phenomenology with clear and measurable manifestations, and is plausibly associated with specific anatomical circuits (
12–
15).
Intermediate phenotypes, sometimes called endophenotypes, are defined as quantifiable, overt measures of discrete brain functions that are state independent, are heritable, cosegregate with the illness in families, and are expressed in some unaffected family members. The intermediate phenotypes may be more closely associated with genes than are complex clinical syndromes, and hence they have utility in genetic and molecular discovery in psychiatry (
16,
17). The strategy of attempting to link genes with intermediate phenotypes has shown modest promise within schizophrenia cohorts (
18–
22). Therefore, we have extended the use of intermediate phenotypes to psychosis as a clinical phenotype, in order to test the hypothesis that intermediate phenotypes are relatively homogeneous in clinical, familial, and phenotypic characteristics across categorical diagnoses and that diagnoses will segregate within families.
The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) was established as a multisite consortium to characterize intermediate phenotypes of the psychosis dimension, with the expectation that some phenotypes would segregate by categorical diagnosis and others would be expressed across the target psychotic disorders (
23). Distinguishing these characteristics can define more biologically homogeneous groups of individuals, generate the molecular, cellular, and systems knowledge that will lead to a biological understanding of psychosis and psychotic disorders (
2), and thereby sharpen conceptualizations about common and distinct aspects of psychosis pathophysiology, risk pathways, and clinical boundaries between psychotic illnesses. The transition from traditional phenomenological classification, as developed by early pioneers in the field of psychiatric nosology (
2,
24,
25), to brain-based disease entities has been helped by the Research Domain Criteria (RDoC) project initiated by the National Institute of Mental Health, an approach that organizes brain disorders as deviations from normal patterns of behavior within the brain’s neuronal networks (
26,
27).
The B-SNIP consortium includes five sites in the United States that were organized to carry out parallel recruitment and phenotyping procedures and that have demonstrated scientific expertise across intermediate phenotypes of psychosis. The process for collecting data on clinical and intermediate phenotypes was standardized across all sites with rigorous quality assurance controls in place to document site-to-site consistency in data acquisition. The B-SNIP consortium has been successful in this intensive psychosis phenotyping effort, as described here in regard to clinical characterization, using frequent face-to-face and audio conferencing and taking advantage of internal technical expertise. In this article, we present the characteristics of the clinical psychosis study group and its analysis, and we report on clinical and demographic characteristics of the patient groups (by categorical diagnoses) and their first-degree relatives across a range of psychotic diagnoses.
Method
B-SNIP Phenotyping Consortium
B-SNIP included sites in Baltimore (G.K.T.), Chicago (J.A.S.), Dallas (C.A.T.), Detroit and Boston (M.S.K.), and Hartford, Conn. (G.D.P.). All sites had preexisting experience with the recruitment, study, and care of psychotic patients with serious mental illness. The sites used identical diagnostic and clinical assessment techniques, and they used similar approaches to recruitment. The study protocol was approved by the institutional review board at each local site. After complete description of the study to the volunteers, written informed consent was obtained. In addition to the clinical characterization, each volunteer underwent comprehensive phenotypic assessment. The Brief Assessment of Cognition in Schizophrenia battery (
28) was used to characterize cognition. Neurophysiologic phenotyping included ocular motor testing with smooth-pursuit and saccade paradigms; resting-state EEG, and auditory-event-related potentials. Structural, diffusion tensor, and resting-state functional brain imaging was conducted. In addition, a blood sample from each volunteer was collected and stored for genetic analysis.
Recruitment
Each site committed to recruit 200 probands with diagnoses of schizophrenia, schizoaffective disorder, or bipolar disorder, at least one of each proband’s first-degree relatives, and 100 healthy comparison subjects. All sites recruited within 20% of the goal. The probands in the study were clinically stable and in a nonacute symptom state. The broad geographical span of B-SNIP facilitated enrichment of the study group by local geographical characteristics. Sites used a combination of newspaper and community advertising for probands and comparison subjects, with the groups similarly recruited across all five sites. Proband recruitment was carried out by dimension (psychosis) within cases of serious mental illness in the schizophrenia-bipolar disorder spectrum, from which all potential participants with a history of psychosis and suspected schizophrenia, schizoaffective disorder, or bipolar disorder were recruited. These probands and comparison subjects are a research sample and neither a clinical nor epidemiological sample; nonetheless, the large study numbers and broad geographical recruitment enhance the generalizability of data from the B-SNIP cohort. This strategy generated a more inclusive study group than is typical in studies focusing on specific disorders, with the aim of having a representative sample of the spectrum of psychotic serious mental illness. Psychosis probands were limited to schizophrenia, schizoaffective disorder, and bipolar disorder because these are the diagnoses with the highest prevalences of psychosis and studying more diagnostic categories was deemed unfeasible as a first approach.
Clinical Characterization of Study Group
Proband volunteers were assessed phenomenologically, with the Hollingshead Index of Social Position, psychiatric and medical histories, a modified family psychiatric history interview (
29), the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P) including the Global Assessment of Functioning (GAF) scale (
30), the Positive and Negative Syndrome Scale (PANSS) (
31), the Young Mania Rating Scale (
32), the Montgomery-Åsberg Depression Rating Scale (MADRS) (
33), the Schizo-Bipolar Scale (
23), and the Birchwood Social Functioning Scale (
34). Relatives were also assessed with the Structured Interview for DSM-IV Personality Disorders (
35) to evaluate personality traits, especially those relevant to the psychosis spectrum, represented by the cluster A personality disorders (
36). Similar evaluation of the healthy comparison subjects assured the absence of a personal history of psychotic or bipolar disorder or recurrent major depressive disorder or a family history of schizophrenia-bipolar spectrum disorders in first- and second-degree relatives.
The extensive clinical information on each volunteer was reviewed in a best-estimate diagnostic meeting with at least two experienced research clinicians, to establish the consensus diagnosis. Cross-site diagnostic conference calls were carried out monthly; they were chaired by two senior primary investigators (C.A.T., M.S.K.) and attended by the 2–4 trained clinical assessors at each site. At study start, there was a face-to-face training session for all raters, with a requirement for reliability above 0.85. Each month, diagnostic conferences were held with in-depth diagnostic discussions. Each year, rater training was repeated to reestablish reliability. A detailed description of the clinical rater training and maintenance, as well as data management and family history methods, is presented in the supplementary methods section in the data supplementappearing with the online version of this article.
Statistical analysis of the sociodemographic and clinical data was done descriptively by using NCSS software (
http://www.ncss.com/software/ncss/). A one-way analysis of variance, with a subsequent Tukey-Kramer multiple comparison test, and the Yates-corrected chi-square test were used, as appropriate. Given the large study group, alpha was set at 0.01 for all statistical analyses.
Discussion
A distinction between the two major psychotic diagnoses, schizophrenia and bipolar disorder, began with Kraepelin (
24,
37) and Bleuler (
25) and persisted throughout the last century. Though Kraepelin himself acknowledged the overlap between these disorders (
37), the dichotomy was reified within APA’s Diagnostic and Statistical Manual editions (
38), even now in the 5th edition. Schizoaffective disorder was first introduced with its own criteria in DSM-III-R, although it was mentioned as a schizophrenia subgroup in previous DSM editions (
9). Previous studies have attempted to test the validity of these categories and, while interesting, have been insufficiently powered (
4,
6) or only conceptual (
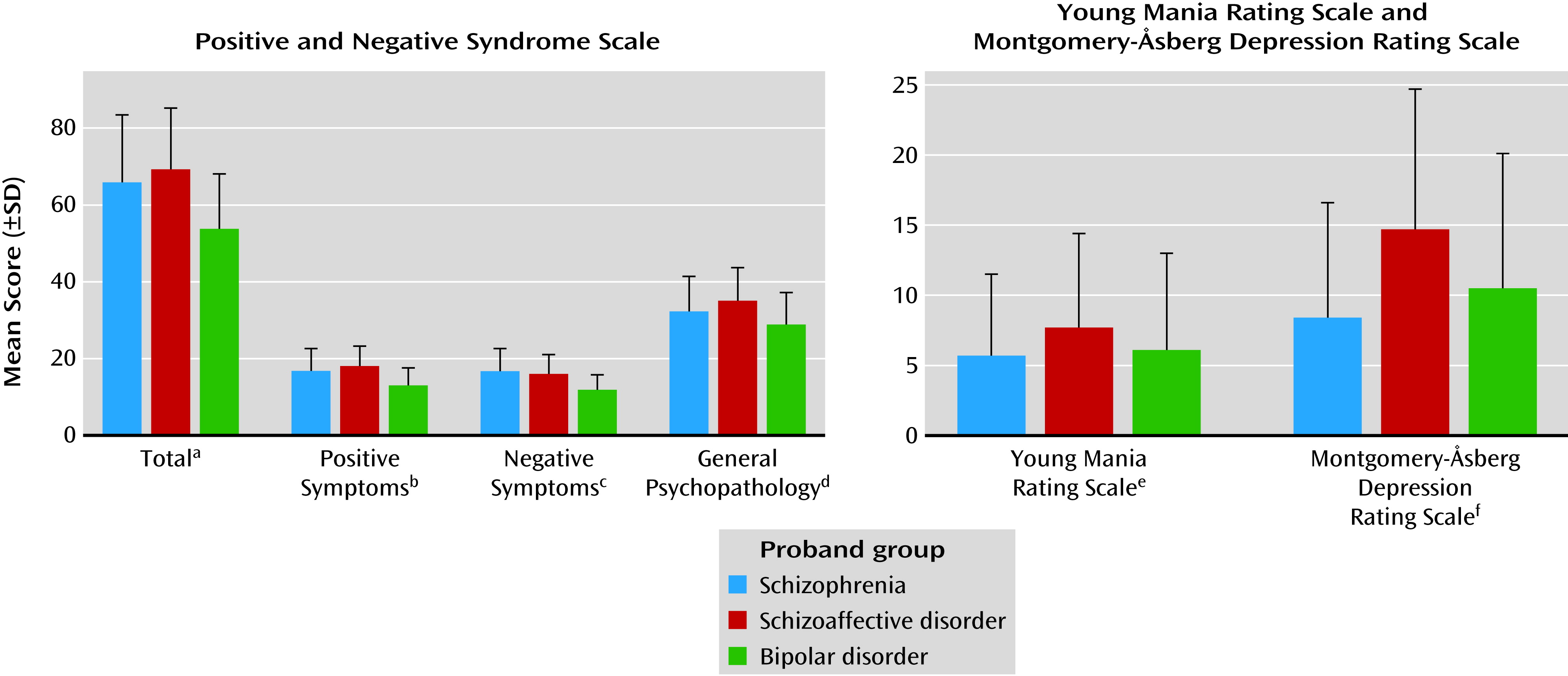
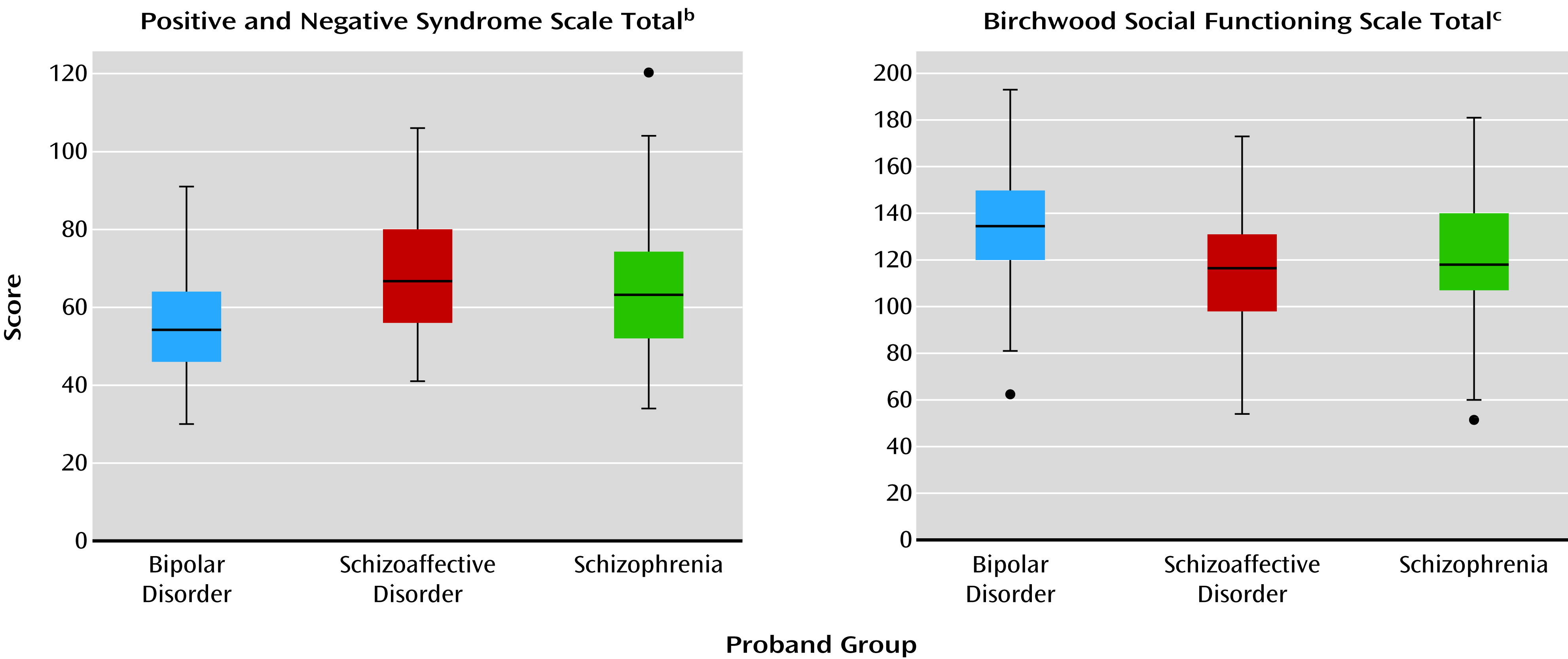
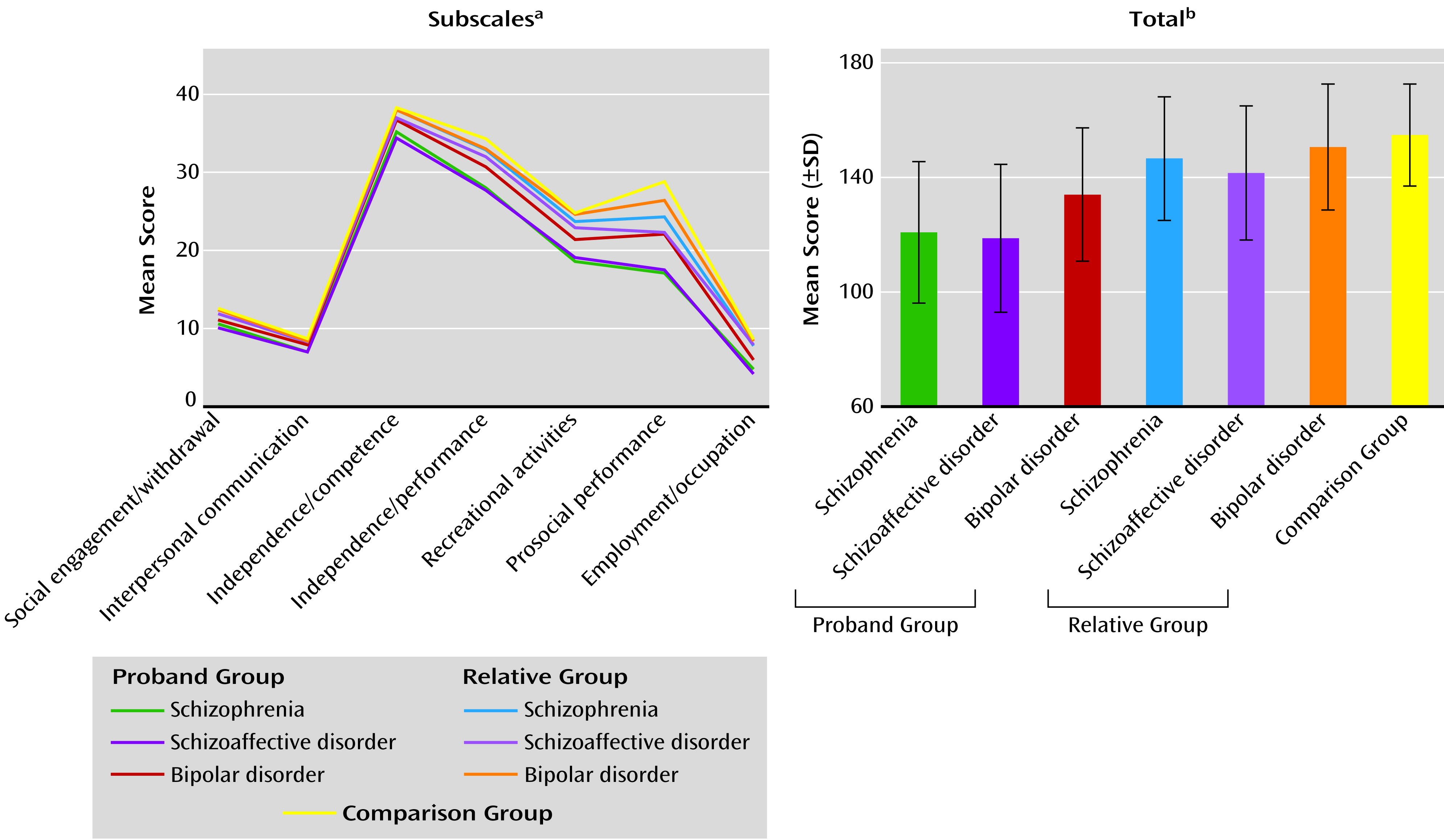
2), leaving questions of commonality and distinctiveness unresolved. In the current study, probands with schizophrenia, contrasted with bipolar probands, had a high degree of overlap in clinical characteristics without categorical distinctions, but they generally showed modestly greater psychosis severity and somewhat poorer psychosocial functioning, even during stable illness periods. Clinical and demographic characteristics of the schizoaffective disorder proband group were often more similar to those of the schizophrenia probands than those of the bipolar group: For example, 1) schizophrenia and schizoaffective disorder proband groups both had lower proportions of Caucasians than the bipolar probands, an observation also reflected in the relative groups; 2) the schizophrenia and schizoaffective disorder probands both had less education than the bipolar proband group, but only the schizophrenia probands had a lower reading level, which reflects education and IQ; 3) PANSS scores (total and all subscales) were higher in both the schizophrenia and schizoaffective disorder probands than in the bipolar probands; and 4) the scores on the social functioning scales were lower in both the schizophrenia and schizoaffective disorder probands than in the bipolar probands. Yet, in some cases, the characteristics of schizoaffective disorder mirrored those of psychotic bipolar disorder: 1) the reports of previous suicide attempts were similarly high among these two proband groups but lower in schizophrenia and 2) the schizoaffective and bipolar disorder proband groups had similarly high proportions of women, whereas the schizophrenia probands had a lower number of women, which is an often-cited characteristic of schizophrenia itself.
The frequency of reported lifetime suicide attempts was disturbingly high in all groups of probands in this B-SNIP cohort, underscoring the immense public health importance of psychosis and its high medical need. It is interesting that the frequency was highest in the probands with schizoaffective disorder. Suicide is among the 10 leading causes of death worldwide; both schizophrenia (hazard ratio=1.87) and mood disorders (hazard ratio=1.72) are associated with suicide (
39). The absolute lifetime risk of completed suicide has been reported to be 7.77% in bipolar disorder and 5.85% in schizophrenia (
40). The evidence that preserved cognitive function is associated with greater suicide ideation (
41) suggests that these suicides are driven by rational considerations along with depressed mood. Both genetic (
42,
43) and molecular (
44) correlates of suicide in schizophrenia and bipolar disorder have been established. The prevalence of this single severe outcome across groups is consistent with the low overall level of psychosocial functioning seen in psychosis.
Epidemiological studies characteristically use family history as the primary measure of genetic liability for disease (
45). Early family studies in schizophrenia found pure schizophrenia lineages in the probands with positive family histories (
46–
48). However, these early observations are now challenged by outcomes from registry-based national cohort studies (
7,
49), which show higher rates of both bipolar disorder and other psychiatric diagnoses within schizophrenia families. Support for this complex inheritance is also seen in population-based studies (
6,
7), extending to the identification of autism (
50) and epilepsy (
51) in schizophrenia lineages. In the current B-SNIP cohort, we verified both “pure” schizophrenia and bipolar disorder pedigrees as well as “mixed” lineages with both schizophrenia and bipolar disorder. Of the probands with schizophrenia, 17.3% had mixed pedigrees (27.0% had schizophrenia-only pedigrees), and of those with bipolar disorder, 25.4% had mixed pedigrees (39.8% had bipolar-only pedigrees). These rates support the existence of genetic overlap between these serious mental illnesses and implicate common genetic mechanisms. Current family and genetic evidence broadens this idea even further, to suggest that many axis I and II psychiatric diagnoses in probands are risk factors for psychotic disorders (
49,
52). Here, among the relatives of the psychosis probands, only 33%−38% were free of any axis I or II diagnosis. These outcomes suggest a high burden of psychiatric morbidity in families with psychosis, a morbidity that is similar across the proband diagnoses.
The B-SNIP study has been motivated by the strong need in serious mental illness research to connect biological characteristics of psychotic illness, through intermediate phenotypes, with diagnostic categories, molecular characteristics, and therapeutic outcomes. The developments in complex disease genetics (
53) and the burgeoning neuroscience knowledge base in our field (
54) provide an excellent opportunity to translate the biological correlates of serious mental illness to disease understanding. The B-SNIP consortium has focused on a single clinical phenotype—psychosis within the schizophrenia-bipolar spectrum—and has attempted to characterize this phenotype biologically. Several meaningful outcomes of such a project can be realized, one of which is to test the distribution of clinical and biological phenotypes across categorical diagnoses, evaluating their biological heterogeneity. Another potential outcome is to determine experimentally whether biologically homogeneous populations emerge out of such a broadly defined clinical phenotype. Not without importance is the goal of understanding how these disparate clinical and intermediate phenotypes covary and segregate, an outcome that could inform their use as clinical biomarkers.
Such transformative goals will need to be rigorously tested in biologically based dense phenotyping protocols, with oversampling, in large numbers of individuals with serious mental illness (
1). The multisite B-SNIP consortium provides sufficient recruiting strength to approach this task, with 933 psychotic probands, along with relatives and healthy comparison subjects. Moreover, the dense phenotyping protocol, with its high cross-site phenotype reliability and single-site expert processing, provides the requisite study group size and phenotype methods to tackle these goals. Previous multisite intermediate-phenotype protocols were models (
18,
19). The intermediate-phenotype reports from B-SNIP will build on this clinical database, contrasting cognitive, neurophysiological, and imaging characteristics of these proband and relative groups, to examine the biological characteristics of these serious mental illnesses within the clinical phenotype of psychosis.