Heavy drinking is common in the United States. In 2010, 23.1% of U.S. individuals age 12 or older reported that, during the prior month, they drank five or more drinks on an occasion, and 6.7% reported doing so on at least 5 days (
1). As the frequency of heavy drinking increases, so does the incidence of a variety of alcohol-related problems, including alcohol use disorder (
2). Despite these risks, only a small fraction of heavy drinkers in the population receives any kind of alcohol treatment, with medications particularly underutilized (
3).
Furthermore, the development of alcohol treatment medications has focused on patients who meet criteria for alcohol dependence, particularly patients whose treatment goal is abstinence, rather than reduced drinking. Some studies of opioid antagonists are exceptions to this (
4–
8).
Although topiramate has shown substantial promise in reducing drinking in patients whose ultimate goal is abstinence (
9,
10), there are no studies, to our knowledge, of its efficacy in treating heavy drinkers whose goal is to reduce their drinking. In an initial single-site 12-week study of topiramate (
9), patients with alcohol dependence who received 300 mg/day of the drug (N=75) had a lower percentage of heavy drinking days than placebo-treated patients (N=75). This effect was replicated in a 14-week multicenter trial of 371 patients (
10). In a study of 61 nontreatment-seeking subjects (
11), individuals were randomly assigned to receive 39 days of treatment with topiramate at a dosage of 200 mg/day, topiramate at a dosage of 300 mg/day, or placebo. Results of the study showed that the frequency of heavy drinking was significantly lower in both topiramate groups than in the placebo group.
Topiramate has multiple pharmacologic effects, including the facilitation of GABAergic function by interacting with a nonbenzodiazepine site on the GABA
A receptor (
12) and antagonism of glutamate activity at AMPA and kainate receptors (
13,
14). Topiramate’s effects on glutamate receptors are most potent and selective for those containing the GluK1 and GluK2 (formerly referred to as GluR5 and GluR6) subunits (encoded by
GRIK1 and
GRIK2, respectively) (
15,
16). Topiramate also blocks voltage-dependent Na
+ and
l-type voltage-gated Ca
++ channels, inhibits carbonic anhydrase, and enhances K
+ conductance (
17).
To identify potential moderators of topiramate response, in a previous study we examined the association of seven single-nucleotide polymorphisms (SNPs) in
GRIK1 to alcohol dependence (
18). One SNP, rs2832407, a C-to-A noncoding substitution, was significantly associated with alcohol dependence, with the C allele being more common in subjects with the disorder. Ray et al. (
19) found that when treated with topiramate,
GRIK1*rs2832407 C-allele homozygotes experienced significantly fewer adverse medication effects than A-allele carriers.
In the present study, we tested two hypotheses: 1) that patients receiving topiramate would show a greater reduction in the number of heavy drinking days and a greater increase in the number of abstinent days than patients receiving placebo and 2) that GRIK1*rs2832407 would moderate the therapeutic response to topiramate. Support for these hypotheses would provide an important option for the personalized treatment of heavy drinking.
Method
Overview
The study was a parallel-group placebo-controlled trial of topiramate in heavy drinkers, all of whom received medical management (
20), a brief psychosocial intervention, at each of nine treatment visits. Patients were randomly assigned to treatment group, and double-blind conditions were maintained throughout the study. Raters were trained in the reliable use of all assessments. The study was conducted in three phases: a 1-week pretreatment assessment period, a 12-week treatment period, and a 9-day medication taper period.
Patients
Inclusion criteria were as follows: age 18–65; an average weekly consumption of ≥24 standard drinks for men and ≥18 standard drinks for women; an explicit goal of reducing drinking to safe levels; ability to read English at a level ≥8th grade; no gross evidence of cognitive impairment; willingness to name a potential locator to ensure follow-up; and written, informed consent to participate. Women of childbearing potential were required to be nonlactating and practicing a reliable method of birth control and to have a negative serum pregnancy test at screening.
Exclusion criteria were the presence of a current, clinically significant physical disease or abnormality on the basis of medical history, physical examination, or routine laboratory evaluation; history of nephrolithiasis; serious psychiatric illness on the basis of history or examination; a current DSM-IV diagnosis of drug (other than nicotine) dependence; and evidence of the likely need for abstinence from alcohol (i.e., current severe alcohol dependence, disorders exacerbated by heavy drinking [e.g., gastritis], self-reported inability to reduce drinking, or current alcohol withdrawal symptoms or a history of severe withdrawal symptoms).
We screened 200 prospective participants in person, of whom 138 (86 men, 62.3%) were randomly assigned to treatment with topiramate (N=67, 48.6%) or placebo (N=71, 51.4%). The CONSORT diagram of the study is presented in the data supplement that accompanies the online edition of this article. The study was initiated at the University of Connecticut Health Center (N=76) and completed at the University of Pennsylvania Treatment Research Center (N=62), and the institutional review boards at both universities approved the study protocol. Patients were paid to complete research assessments.
Procedures
Individuals were recruited through advertisements. An initial telephone screening interview was followed by an in-person visit, at which time patients provided written, informed consent to participate, and they underwent a history, physical examination, routine laboratory testing, and a urine drug screen, as well as pregnancy testing (for female patients).
Prior to randomization, patients completed questionnaires and were administered research interviews by a trained research evaluator. A nurse then administered the first medical management session and dispensed the study medication. We balanced the medication groups on age, sex, and frequency of drinking days and heavy drinking days during pretreatment using urn randomization, and we stratified the randomization for patients receiving antidepressants.
During the first 6 weeks of treatment, patients were seen weekly for medication titration, followed by three biweekly visits. At each visit, the patients’ breath alcohol concentration, weight, and vital signs were measured; patients completed questionnaires; and the research nurse elicited information on concurrent medications, the occurrence of adverse events, and protocol adherence and delivered the medical management intervention (
20). At each visit, patients were interviewed to measure drinking and medication use since the last visit. The nurse compared self-reported adherence with the number of capsules returned and discussed discrepancies with the patients to resolve them. At the end of treatment, patients again completed questionnaires and were interviewed by the research nurse and the research evaluator.
Study Treatments
Counseling.
The medical management manual we used focuses on medication adherence and treatment participation through education and support; it was modified to be consistent with a goal of sensible drinking. The initial session included a review of the results of the initial evaluation and a discussion of sensible drinking limits using a World Health Organization guide on sensible drinking (
21), as well as a rationale for and information about pharmacotherapy and the importance of medication adherence. At subsequent sessions (20–30 minutes), the nurse briefly assessed the patients’ drinking, monitored medication adherence, and made recommendations related to both. Based on guidelines for nonhazardous drinking (
22), men were advised to consume no more than three standard drinks per day and 12 standard drinks per week, and women were advised to consume no more than two drinks per day and eight drinks per week. Thus, patients were counseled both to avoid heavy drinking days and to increase the number of abstinent days. Sessions were audiotaped and reviewed, and feedback was provided to nurses to ensure consistency in their delivery.
Medication.
We selected a maximal daily dosage of 200 mg of topiramate based on evidence of its efficacy (
9–
11) and to limit the adverse effects associated with a higher medication dosage (
10). Topiramate treatment was initiated at a dosage of 25 mg at bedtime and at weekly intervals was increased as follows: 50 mg at bedtime, then 25 mg in the morning and 50 mg at bedtime, then 50 mg twice daily, then 50 mg in the morning and 100 mg at bedtime, and, finally, 100 mg twice daily. Placebo and topiramate were encapsulated and indistinguishable from one another. Dosage reductions or a delay in the increase in dosage was used to manage adverse effects.
Assessments
Laboratory assessments.
Laboratory assessments included urinalysis and urine toxicology testing, a complete blood count, γ-glutamyl transpeptidase concentration measurement, and a chemistry panel (including electrolytes, liver enzymes, and bilirubin). Measurement of electrolyte levels was repeated at the study midpoint to screen for metabolic acidosis. Measurement of γ-glutamyl transpeptidase concentration was repeated at the midpoint and at the end of treatment to validate self-reported drinking.
Psychological and behavioral assessments.
Psychological and behavioral assessments are listed below.
1) Sociodemographic/clinical information: Marital status, educational and occupational information, medical history, and substance abuse treatment history were included.
2) Psychiatric diagnosis: The Structured Clinical Interview for DSM-IV-TR Axis I Disorders (
23) was used to classify patients according to the presence or absence of standard psychiatric disorders according to DSM-IV criteria (
24).
3) Alcohol use patterns: The timeline follow-back method (
25) was used to estimate the number of abstinent days and heavy drinking days during the 90-day pretreatment period and at each treatment visit.
4) Alcohol-related problems: The Short Index of Problems (
26), a 15-item single-factor measure of alcohol-related problems (
27), was administered at baseline and study endpoint.
5) Depressive symptoms: The Beck Depression Inventory (
28), a 21-item self-report measure of depressive symptoms (score=0–63), was administered at baseline. It was repeated at every study visit for patients who were receiving an antidepressant or whose depression score was elevated at baseline.
Genotyping Procedure
DNA was extracted from whole blood using the PureGene kit (GentraSystems, Minneapolis). We genotyped rs2832407 using the TaqMan SNP genotyping assay (Life Technologies, Grand Island, N.Y.). All genotypes were obtained in duplicate with consistent results.
Statistical Analysis
Descriptive statistics included means and standard deviations for continuous variables (group differences analyzed using t tests) and percentages for categorical variables (group differences analyzed using chi-square). Factorial models crossing treatment assignment with a three-level genotype for group were analyzed using the general linear model for continuous variables and logistic regression for dichotomous categorical variables.
Timeline follow-back data.
Drinking data were aggregated to the weekly level. The number of days per week of heavy drinking (i.e., four or more drinks in a day for women and five or more drinks in a day for men) and of abstinence were the primary outcomes. Generalized linear mixed models with a binomial distribution and logit link function were used to examine medication group differences in changes in these outcomes during treatment. The models included 1) fixed effects for medication group, week, and interaction between medication and week and 2) a random effect for intercept. “Week” was recoded by subtracting the number of weeks of the study (
12) so that the test of treatment compared the groups at the conclusion of the study (week 12), rather than at baseline. The interaction term tested for different rates of change in the outcome during the study.
Two sets of analyses of the number of heavy drinking and abstinent days were conducted. First, an intent-to-treat analysis included all 138 patients. In addition to examining changes in drinking over time, we conducted a responder analysis that examined the number of patients in each group with no heavy drinking days during the last 4 weeks of treatment, consistent with the approach recommended by the Food and Drug Administration (
29). Second, we conducted a pharmacogenetic analysis that was limited to self-identified European American patients (N=122) because of substantial population differences in rs2832407 allele frequency. Initially, we used the three-level genotype for rs2832407 by adding it to the linear mixed analysis. We then combined the AA and AC groups, comparing them with the CC group as a dichotomous genotype.
Timeline follow-back data were available for 92.4% (SD=22.7) of the 84 days of treatment (92.9% [SD=20.9] for topiramate patients and 91.9% [SD=24.5] for placebo patients). To examine the effect of missing data, multiple imputation using a Markov Chain Monte Carlo single chain method based on patient baseline characteristics and weekly drinking was employed to create 10 imputed data sets. Models were rerun on the imputed data sets using SAS Proc Mianalyze (SAS Institute, Cary, N.C.).
Measures to validate drinking outcomes.
The γ-glutamyl transpeptidase concentrations were analyzed at the study midpoint and endpoint. Because of severe positive skewness and kurtosis, the values were log transformed. The Short Index of Problems score was analyzed using analysis of covariance, controlling for the pretreatment score.
Results
DSM-IV Diagnoses and Antidepressant Treatment
Although a DSM-IV diagnosis of current alcohol dependence was not an inclusion criterion, the vast majority of patients (92.5% of topiramate patients and 91.5% of placebo patients) met criteria for the diagnosis. Despite a high lifetime prevalence of major depression (
Table 1), only three patients receiving topiramate and five patients in the placebo group met current criteria for an anxiety or depressive disorder. A total of 23 patients were receiving antidepressants at the time of randomization (16.4% of topiramate patients and 16.9% of placebo patients).
Treatment Completion
Treatment completers were those who completed 12 weeks of treatment. Overall, 117 patients (84.9%) completed treatment (topiramate patients: N=55, 82.1%; placebo patients: N=62, 87.3%; χ2=0.73, df=1, p=0.39). The two medication groups were comparable on the number of weeks of treatment received (topiramate patients: mean=10.9 weeks [SD=2.6], placebo patients: mean=11.1 weeks [SD=2.7]). Among European Americans, there was no main effect of genotype group or interaction of genotype group with medication group on treatment weeks.
Medication Adherence and Maximal Dosage Achieved
Using self-reports, with verification by capsule counts, there was a high rate of adherence in both medication groups (placebo patients: mean=91.1% of daily doses [SD=24.7]; topiramate patients: 89.4% of daily doses [SD=23.1]). The finding of placebo patients taking a higher dosage of the study medication (equivalent dosage=187.7 mg [SD=43.1]) than topiramate patients (173.5 mg [SD=45.6]) fell short of statistical significance (F=3.53, df=1,136, p=0.06). Among European Americans, there was no difference in maximal dosage by genotype group or the interaction of genotype group with medication group.
Main Effects of Topiramate
Demographic and pretreatment clinical measures.
The study sample consisted predominantly of middle-aged, European American, married, employed men, with an average of 3 years of college (
Table 1). During pretreatment, patients drank alcohol approximately 6 days per week and drank heavily 5 days per week. The only pretreatment demographic or clinical measure on which the groups differed significantly was age. Placebo patients were approximately 3.5 years older than topiramate patients. We included age as a factor in the analyses, as described below. There were site differences with regard to demographic characteristics. Patients from Connecticut were predominantly European American (97%), whereas in Philadelphia there were fewer European Americans (77%) and more African Americans (18%) (χ
2=14.60, df=3, p=0.002). In addition, the Connecticut patients were significantly (χ
2=7.36, df=1, p=0.007) more likely to be married (71%) than the Philadelphia patients (48%) and to work full-time (78% compared with 48%, χ
2=13.38, df=2, p=0.001). We examined site as a factor in the analyses, as described below.
Heavy drinking days.
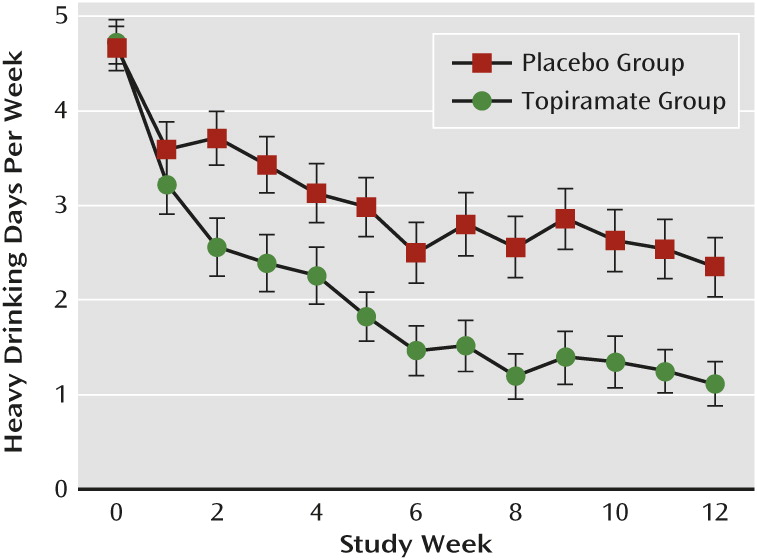
There was a significant main effect of medication group, with topiramate patients reducing heavy drinking more than placebo patients (F=23.37, df=1, 1399, p<0.001), and an interaction of medication group and treatment week (F=19.91, df=1, 1399, p<0.0001), with topiramate patients decreasing heavy drinking more rapidly than placebo patients. By the last week of treatment, the odds of experiencing a heavy drinking day in the placebo group was 5.33 times (95% confidence interval [CI]=1.68–7.28) that of the topiramate treatment group (
Figure 1).
The number of patients with no heavy drinking days during the last 4 weeks of treatment in the topiramate group (N=24, 35.8%) was more than double that found in the placebo group (N=12, 16.9%) (odds ratio=2.75, 95% CI=1.24–6.10).
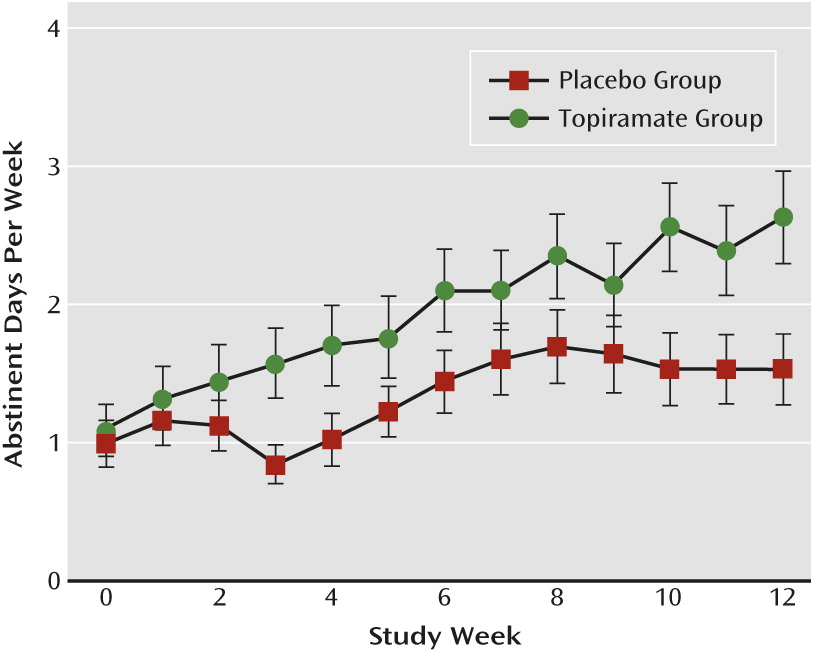
Abstinent days.
There was a main effect of medication group (F=4.63, df=1, 1398, p=0.03), with topiramate patients reporting more abstinent days than placebo patients. There was also a significant interaction of medication group and treatment week (F=6.26, df=1, 1398, p=0.01). Topiramate patients increased the number of abstinent days per week more rapidly than placebo patients. By the last week of treatment, the odds of abstaining from drinking among topiramate patients was 2.57 times (95% CI=1.13–5.84) that of placebo patients (
Figure 2).
γ-Glutamyl transpeptidase concentrations.
There was a significant medication group-by-time interaction (F=3.44, df=2, 241, p=0.03). Topiramate-treated patients had a significantly greater decline in γ-glutamyl transpeptidase concentrations than placebo patients. Although concentrations were equivalent at baseline (topiramate patients: N=67, mean=65.9 IU/L [SD=91.5]; placebo patients: N=71, mean=56.1 IU/L [SD=71.6]), there was a near-significant difference at midpoint (topiramate patients: N=59, mean=37.6 IU/L [SD=36.7]; placebo patients: N=64, mean=50.1 IU/L [SD=64.8]; p=0.06), and a significant difference at endpoint (topiramate patients: N=58, mean=36.3 IU/L [SD=40.2]; placebo patients: N=63, mean=47.9 IU/L [SD=52.1]; p=0.01).
Short Index of Problems score.
Controlling for baseline scores, there was a significant difference in Short Index of Problems scores at the study endpoint (Δ=7.9 for topiramate patients, from 14.9 [SD=8.6] at randomization to 7.0 [SD=7.2] at endpoint, and Δ=4.4 for placebo patients, declining from 15.5 [SD=6.7] at randomization to 11.1 [SD=7.5] at endpoint [F=11.42, df=1, 128, p=0.001]).
Moderation of the Effects of Topiramate by rs2832407
The demographic and pretreatment clinical features as a function of both genotype and treatment groups for the European American subsample that was the focus of the pharmacogenetic analyses are summarized in
Table 2. Consistent with the finding in the intent-to-treat sample, placebo-treated patients were significantly older than topiramate-treated patients. No other demographic or clinical features differed significantly among the groups.
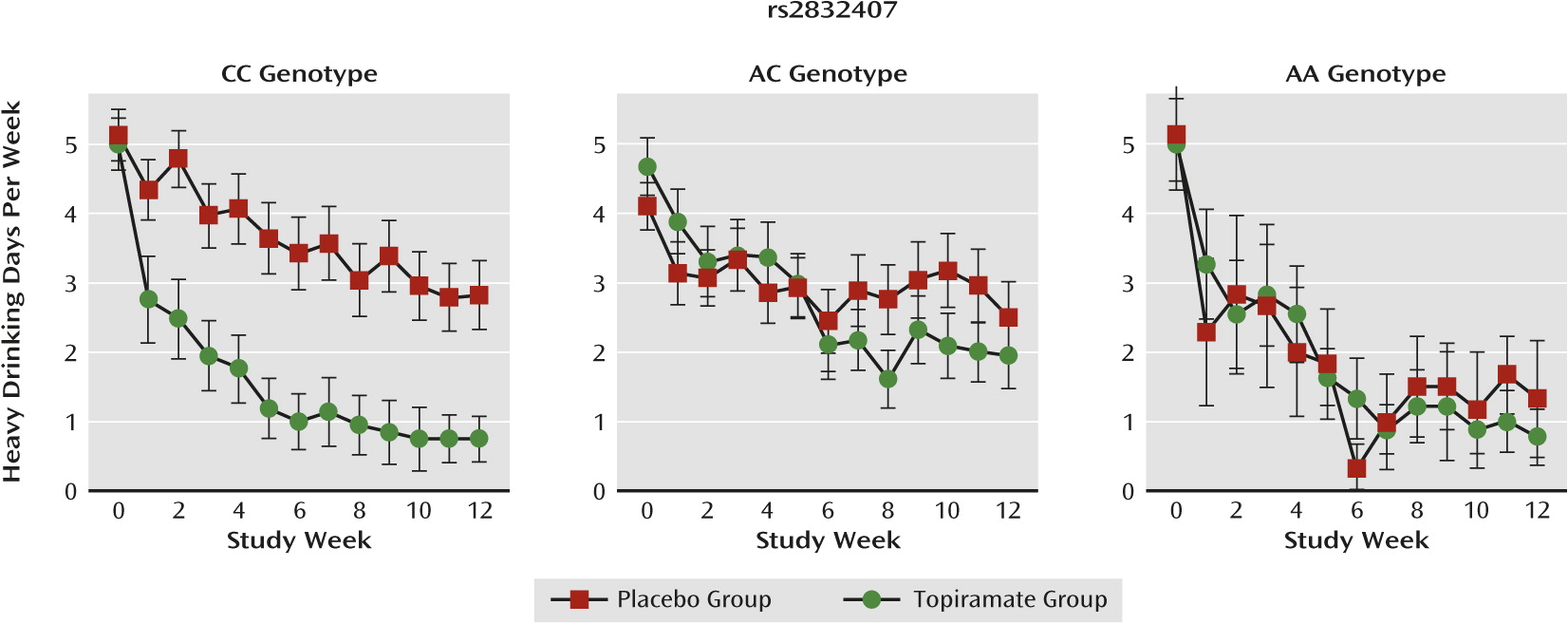
Heavy drinking days.
There was a significant medication group-by-genotype interaction (F=5.50, df=2, 1227, p=0.004) on heavy drinking days. As shown in
Figure 3, topiramate was efficacious only in patients with the CC genotype. In follow-up comparisons in patients with the CC genotype, topiramate reduced heavy drinking days significantly more than placebo (F=23.81, df=1,1228, p<0.001), whereas in A-allele carriers the difference between topiramate and placebo was not significant.
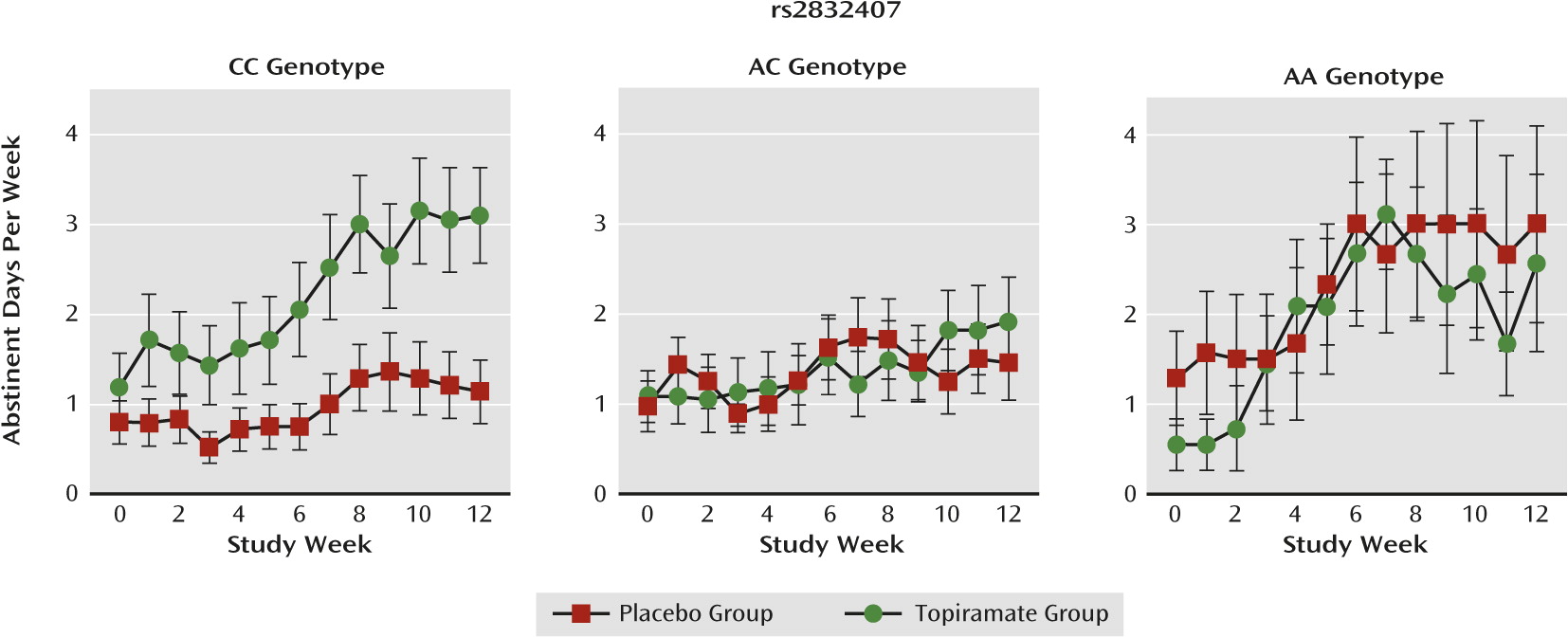
Abstinent days.
For abstinent days, the interaction of medication group-by-genotype group was not statistically significant. Nonetheless, as with heavy drinking days, the effect of topiramate appeared to be limited to the C-allele homozygotes (
Figure 4), and a contrast between topiramate and placebo within C-allele homozygotes (compared with A-allele carriers) was significant (F=4.08, df=1, 1228, p=0.04).
Effect of age, site differences, antidepressant treatment, and missing data.
Age, treatment site, and antidepressant treatment were not associated with the drinking outcomes, and including them in the models did not substantially alter the findings. Furthermore, analyses based on multiple imputation of missing data yielded findings that were wholly consistent with the primary analyses.
Adverse Effects
Topiramate patients reported significantly more adverse events (mean=5.5 [SD=3.1]) than placebo patients (mean=3.0 [SD=2.5]; F=29.0, df=1, 135, p<0.001). Although approximately two-thirds of the adverse events were rated as mild, topiramate patients reported more moderate or severe events (mean=1.8 [SD=1.3]) than placebo patients (mean=0.4 [SD=0.7]) (F=62.2, df=1, 135, p<0.001). The adverse events that occurred in at least 10% of the patients and the number of patients from each group that experienced the event are summarized in Table S1 of the online
data supplement. A significantly greater number of topiramate patients reported numbness/tingling, change in taste, loss of appetite, weight loss, difficulty concentrating, and difficulty with memory; these are all adverse effects that have been associated commonly with topiramate treatment (
9,
10).
Discussion
In this study, we examined the efficacy of topiramate at a maximal daily dose of 200 mg in patients whose goal was to reduce their drinking, rather than to become abstinent from alcohol. We found significantly greater effects of topiramate than placebo in reducing heavy drinking days and increasing abstinent days. Both γ-glutamyl transpeptidase concentration, an objective measure of heavy drinking, and Short Index of Problems scores, a measure of alcohol-related problems, were consistent with the self-reported drinking data. This evidence of efficacy compares favorably with the findings from two previous studies that compared topiramate 300 mg with placebo to promote abstinence (
9,
10). Furthermore, the findings reported in the present study are consistent with those of a study of nontreatment-seeking heavy drinkers, in which both 200 mg and 300 mg of topiramate reduced the frequency of heavy drinking more than placebo (
11).
Of particular note, we observed that rs2832407, a noncoding intronic SNP in
GRIK1, moderated topiramate’s effects on heavy drinking days. Although a similar pharmacogenetic effect was seen for the number of abstinent days, it did not reach statistical significance. A larger sample could yield a significant moderator effect on this outcome as well. In a previous analysis of moderation by this SNP (
19), patients with the CC genotype had lower plasma concentrations of topiramate and fewer adverse effects of the medication than A-allele carriers. We did not measure topiramate plasma concentrations but found no effect of rs2832407 on adverse effects produced by topiramate (see the online
data supplement).
Although the functional effects of rs2832407 are unknown, the SNP is located 802 base pairs upstream of a
GRIK1 antisense transcript that overlaps with exon 9. Analysis of data from the 1,000 Genomes Project (
30) shows that it is in near-complete linkage disequilibrium (r
2=0.99) with rs363431, which maps to the antisense transcript in
GRIK1. Furthermore, ENCODE data show that rs2832407, which is a C-to-A substitution, lies within a CpG island (
31). Thus, one possible mechanism for the observed moderating effect of rs2832407 is that the polymorphism, or another
GRIK1 polymorphism that is linked to it, affects the level of expression of
GRIK1 mRNA, reducing the number of GluK1-containing kainate receptors and the effect of topiramate.
The high rate of treatment completion was a strength of this study. In contrast to the multicenter study by Johnson et al. (
10), in which a significantly larger proportion of topiramate-treated patients than placebo patients discontinued treatment prematurely, we found no evidence of differential attrition. The observed reduction in heavy drinking days, particularly in patients with the CC genotype at rs2832407, to less, on average, than one heavy drinking day per week is clinically important, since the frequency of heavy drinking is correlated with a variety of alcohol-related negative consequences (
32–
34). In addition to requiring replication in a larger sample, the effects of topiramate and the moderating effects of rs2832407 require evaluation in populations other than European Americans. Together, these efforts will help to personalize the pharmacological treatment of heavy drinking.