Cigarette smoking is a leading cause of premature death and disease in the United States and in developing countries throughout the world (
1). According to recent studies, smoking is even more lethal than previously believed, with continuing smokers experiencing nearly three times the death rate of nonsmokers (
2). Smoking cessation demonstrably reduces the risk of smoking-related mortality, and the younger a smoker is when he or she quits, the greater the benefit of quitting (
3). Unfortunately, with current pharmacotherapies, which include nicotine replacement therapy, varenicline, and sustained-release bupropion, success rates remain low, with less than 25% of smokers remaining abstinent 1 year after treatment (
4).
To increase the efficacy of smoking cessation treatment, we have developed and validated an adaptive treatment approach designed to provide each smoker with the treatment most likely to succeed, while minimizing risks and side effects. According to this strategy, smokers initially receive nicotine patch treatment starting 2 weeks before a target quit date. The initiation of nicotine replacement therapy before the quit date has been shown to be well tolerated, and several studies have found it to enhance continuous smoking abstinence (
5–
7). Moreover, it allows the early identification of positive responders to nicotine replacement, based on the extent to which ad lib smoking declines in the first week of prequit nicotine patch treatment. We found in previous studies (
8,
9) that smokers who did not decrease their smoking by more than 50% in the first week had a much lower abstinence rate after the quit date than smokers who reduced their smoking by more than 50%. Using this early marker of nicotine patch response to guide subsequent treatment, we were able to prevent approximately 10% of treatment failures among patch nonresponders by modifying the treatment before the target quit date, either by switching to varenicline or augmenting nicotine replacement therapy with bupropion (
9).
Given that varenicline and bupropion have different mechanisms of action, it is reasonable to hypothesize that their therapeutic effects might be additive. This rationale was supported by recent open-label investigations suggesting that combination treatment with varenicline and bupropion was well tolerated and potentially highly efficacious (
10,
11). Inasmuch as combination treatment with two drugs that carry FDA black box warnings may face significant hurdles in becoming a first-line therapy, a more feasible approach may be to evaluate the usefulness of this combination treatment for smokers who are not likely to succeed in quitting with nicotine patch treatment alone. In the present study, we evaluated whether combination treatment with varenicline and bupropion is more efficacious than varenicline alone as a rescue treatment for nicotine patch nonresponders.
Method
Study Design
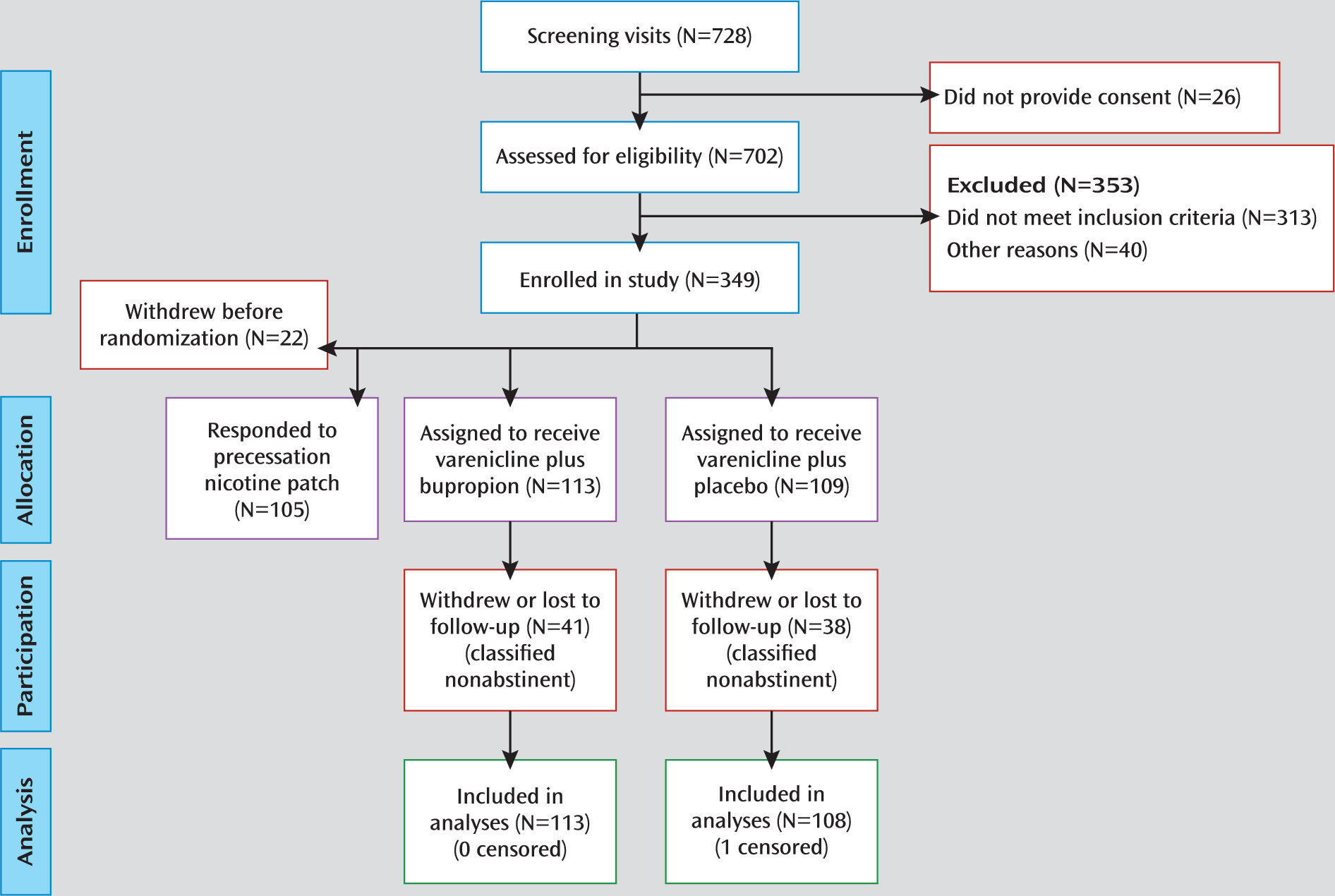
The study was a double-blind, parallel-arm adaptive treatment trial, in which early response to nicotine patch treatment was assessed in a prequit phase, after which nicotine patch nonresponders who failed to show a decrease of >50% in ad lib smoking (assessed using expired-air CO) were randomly assigned to receive either varenicline plus bupropion (N=113) or varenicline plus placebo (N=109). (The nicotine patch prequit responders were entered into a separate study to explore combination nicotine replacement therapy treatment, the results of which will be reported elsewhere.)
Study Procedures
The study was approved by the Duke University Medical Center Institutional Review Board. Adult smokers expressing a desire to quit smoking were recruited through newspaper, radio, and television advertisements. To be eligible, participants had to be 18–65 years of age, report smoking an average of ≥10 cigarettes per day for 3 cumulative years, have an expired-air CO level ≥10 ppm, and have no exclusionary features on history, physical examination, or laboratory evaluation (see the data supplement that accompanies the online edition of this article). Participants provided written informed consent after receiving a complete description of the study, and they were compensated up to $330 for study participation.
After screening and enrollment in the study, participants were seen at our research center weekly for 2 weeks before the quit date and at four sessions held 1, 3, 7, and 11 weeks after the quit date. Brief support (<15 minutes) was provided at each session, and clinical trial materials were dispensed. Smoking diaries and measures of expired-air CO were collected at each session.
After the first week of prequit nicotine patch treatment, all patch nonresponders were randomly assigned to receive varenicline along with either sustained-release bupropion tablets or placebo tablets that were identical in appearance. The recommended dosing titration schedule was used for both varenicline (0.5 mg once daily on days 1–3; 0.5 mg twice daily on days 4–7; and 1 mg twice daily through 12 weeks) and bupropion (150 mg daily for 3 days, then 150 mg twice daily through 12 weeks).
Initial nicotine patch dosing was based on initial expired-air CO reading; participants with CO levels >30 ppm at baseline received 42 mg/day (two 21-mg/day patches), and the remaining participants received a single 21 mg/day patch. In the 21 mg/day condition, one 21-mg patch was applied each morning; in the 42 mg/day condition, a 21-mg patch was applied each morning and a second patch at noon (which has been found to reduce the likelihood of nausea). This personalized dosing regimen was based on previous research suggesting that heavy smokers may not receive adequate nicotine replacement with a 21-mg patch (
12,
13).
Dosage reductions for medications were allowed in the event of adverse effects. Adverse effect ratings were collected using 7-point rating scales.
Statistical Analysis
Treatment groups were initially compared on demographic and smoking history variables, using analysis of variance, to identify potential confounding factors.
To evaluate the hypothesis that combination treatment with varenicline and bupropion would enhance abstinence rates compared with varenicline alone, logistic regression was used to compare the two treatment groups on the primary outcome of continuous abstinence at end of treatment (weeks 8–11 after the target quit date). Abstinence was identified by self-report of no smoking confirmed by expired-air CO levels ≤10 ppm. A secondary outcome measure was point (7-day) abstinence at 6 months (self-reported abstinence confirmed by expired-air CO level at the follow-up).
In an intent-to-treat analysis, participants who withdrew from the study or were lost to follow-up were classified as nonabstinent, consistent with previous reports suggesting that dropouts are very likely to be smoking (
14). However, if not all dropouts are smoking, then this failure imputation for study dropouts might lead to reduced estimates of variance, biased conclusions, or elevated rates of type I error (
15). Similar problems occur with standard imputation methods that erroneously assume that data are missing at random with respect to treatment outcome. In our sample, a high proportion of dropouts reported at the session immediately preceding withdrawal that they had been smoking, which suggests that the missing-at-random assumption was tenuous. Accordingly, we estimated two logistic regression models to model the probabilities of dropping out and of smoking based on individual participant characteristics and smoking status at previous time points, carrying the associated coefficients forward into separate imputation models (using PROC MI in SAS, version 9.2 [SAS Institute, Cary, N.C.]). A third set of imputation models was estimated based on procedures described by Hedeker et al. (
15), using a variety of assumed odds ratios to model the probability of smoking among study dropouts. Results from these and the latter two imputations were combined into single estimates as described by Schafer (
16) (using PROC MIANALYZE). A sensitivity analysis was then conducted to determine how robust the conclusions were to the different assumptions underlying these various imputation regimes (including last observation carried forward and missing=smoking).
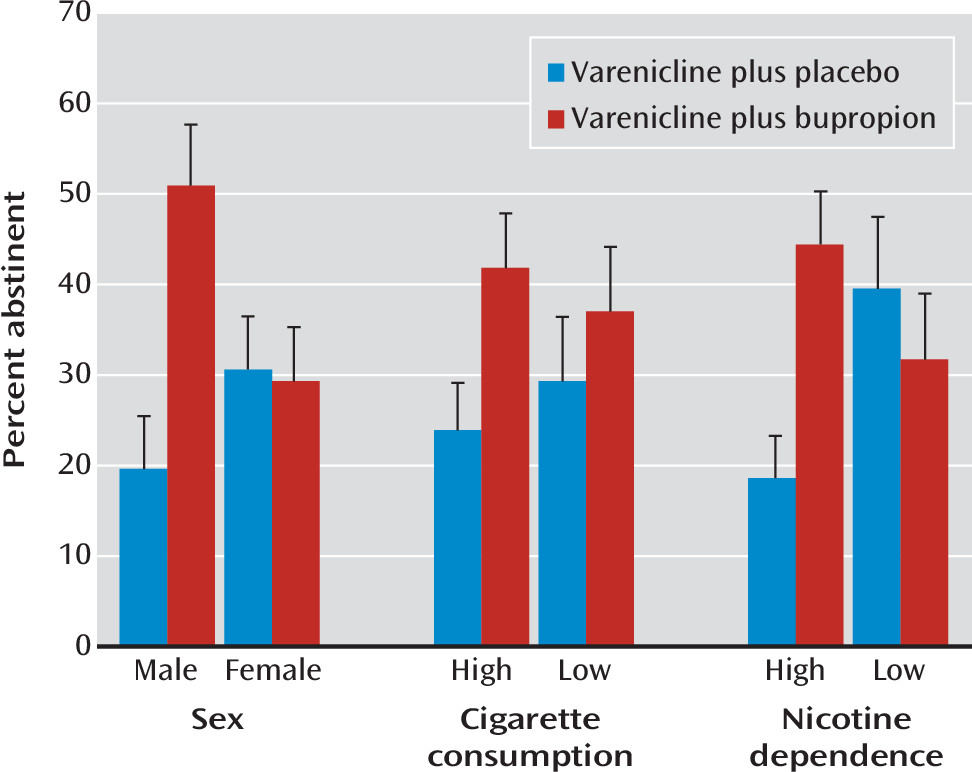
Demographic variables, including sex, were examined for potential moderating effects on treatment outcome. Additionally, we sought to replicate two specific findings reported in a recent article published after the completion of the present study. Ebbert et al. (
17) reported that levels of nicotine dependence and cigarette consumption moderated the efficacy of combination treatment with varenicline and bupropion, with more highly dependent smokers (a score >5 on the Fagerström Test for Nicotine Dependence) and heavier smokers (≥20 cigarettes/day) showing a greater therapeutic response to the combination treatment. The same baseline variables (and their interaction with treatment condition) were therefore included in our logistic regression models to assess their potential association with treatment outcome.
Adverse effects were tabulated according to severity and compared between treatments using chi-square tests.
Discussion
The study results showed an overall benefit of adding bupropion treatment to varenicline for nicotine patch nonresponders. Moreover, male smokers and those with high levels of nicotine dependence appeared to have a greater therapeutic response. For these smokers, end-of-treatment abstinence rates among those in the combination treatment condition were more than double those of smokers in the varenicline-plus-placebo condition. This end-of-treatment difference was sustained, although to a lesser extent, at the 6-month follow-up point. In contrast, no statistically significant difference between the two treatments was observed in female smokers or those with lower levels of nicotine dependence, although the 95% confidence intervals for the odds ratios cannot preclude an effect in these subgroups.
Ebbert et al. (
17), in their recently published study evaluating the efficacy of combination treatment with varenicline and bupropion, also reported a significantly higher end-of-treatment abstinence rate for combination treatment with varenicline and bupropion than for varenicline plus placebo, as well as a greater therapeutic effect in smokers with higher dependence levels. No statistically significant sex-by-treatment interaction was detected. However, the design of that study differed in an important respect from ours: all participants were randomly assigned to the two treatments, as opposed to randomizing only the nicotine patch nonresponders.
What potential mechanisms might explain why male smokers who do not achieve sufficient smoking reduction during prequit nicotine patch treatment benefit from receiving combination treatment with varenicline and bupropion? In animal models, some studies have found that males exhibit a greater increase in nicotinic receptor up-regulation than females after chronic nicotine administration (
18). Moreover, a recent brain imaging study in smokers found that men, but not women, showed an up-regulation of nicotinic receptors in the striatum, a region important for drug addiction and reward (
19). The extent of nicotinic receptor up-regulation, in turn, has recently been shown to predict smoking relapse (
20). Therefore, it is conceivable that bupropion and its metabolites, acting as nicotinic receptor antagonists (
21), counteract the adverse effects of nicotinic receptor up-regulation in male smokers.
Additionally, sex differences have been reported in striatal dopamine D
2/D
3 receptor availability (
22), with male smokers showing a down-regulation compared with male nonsmokers and female smokers. Males have also been found to exhibit greater dopamine release in response to drugs of abuse (
23), which suggests that they may be more dependent on the dopaminergic stimulation obtained from smoking. Varenicline has been shown to influence striatal dopaminergic transmission, both by enhancing dopamine release (
24) and by up-regulating dopamine D
2/D
3 receptors (
25). However, varenicline, acting on the same receptors as nicotine itself, may provide insufficient dopamine release for nicotine patch nonresponders. Bupropion, in contrast, enhances dopamine transmission through a distinct mechanism, by blocking dopamine reuptake (
26). Thus, male smokers who do not respond adequately to nicotine patch treatment may benefit more than female smokers from the action of bupropion on dopamine transmission.
The interaction of treatment with baseline nicotine dependence cannot be attributed to the exclusion of nicotine patch responders, given that the interaction was also observed in the Ebbert et al. (
17) study, which did not exclude nicotine patch responders. The interaction is also difficult to explain in terms of the two component treatments, as nicotine dependence level has not generally been reported as a moderating factor in studies that have evaluated varenicline or bupropion individually (
27,
28). However, the index of efficacy in these studies has been the comparison of each agent to placebo rather than comparing combination treatment to varenicline plus placebo. Mechanisms underlying the interaction of combination treatment and dependence level will require further investigation.
In addition to showing superior efficacy of combination treatment in specific subpopulations of smokers, our results further validate an adaptive treatment model, in which smokers receive a week of prequit nicotine patch treatment, and based on their reduction in ad lib smoking, either remain on the patch or receive adaptive modifications of treatment. The previous randomized controlled trial in our center (
9) showed that approximately 10% of smokers who had a poor initial response to prequit nicotine patch treatment could be rescued by adaptive changes in treatment. In that study, the rescue treatments were varenicline alone or augmentation of nicotine patch treatment with bupropion. The present study extends these results by showing that combination treatment with varenicline and bupropion is an efficacious rescue treatment for male smokers and for highly dependent smokers.
Strengths of the study include the randomized, placebo-controlled design and the use of an adaptive treatment regimen. The study also had limitations. It did not evaluate whether combination treatment with varenicline and bupropion might prove more efficacious than varenicline as an initial treatment for nicotine patch responders; however, our rationale was to avoid the additional risks and side effects of varenicline and bupropion for nicotine patch responders, who have a reasonable chance of quitting using nicotine replacement alone (
8). Another limitation was the use of a tailored dosage of precessation nicotine patch therapy and the concurrent use of nicotine replacement and cigarettes during the precessation period, which deviated from current product labeling in the United States. However, the FDA recently issued more liberal guidelines regarding product labeling with respect to concurrent use of nicotine replacement by individuals who continue to smoke (
29). Additionally, precessation nicotine patch use is approved in several other countries.
In summary, based on the magnitude of enhancement in abstinence rate and an excellent safety and tolerability profile, the results of this study support the use of combination treatment with varenicline and bupropion for male smokers and highly dependent smokers who do not respond with sufficient smoking reduction during the first week of prequit nicotine patch. Future research should investigate potential biobehavioral mechanisms that may account for the differences in treatment response across subpopulations of smokers.