Impaired working memory performance, a core component of cognitive dysfunction in schizophrenia, is associated with altered activity of the dorsolateral prefrontal cortex. The alterations are thought to be due at least in part to disturbances in GABA neurotransmission (
1). For example, mRNA (
2–
8) and protein (
9,
10) levels of the 67-kDa isoform of glutamic acid decarboxylase (GAD67), a key enzyme for cortical GABA synthesis, have been consistently reported to be lower in the dorsolateral prefrontal cortex of subjects with schizophrenia. The frequency with which lower cortical GAD67 levels has been observed in schizophrenia suggests that it is a conserved feature of the disease process. In addition, other observations suggest that lower cortical GAD67 levels are not a consequence of illness chronicity (
11) or of other factors that frequently accompany schizophrenia (
9). Since GAD67 expression is heavily regulated by neuronal or network activity (
12,
13), disease-related alterations in activity-dependent regulatory factors may contribute to lower GAD67 levels (
14).
One activity-dependent regulatory factor that may regulate GAD67 expression is the immediate early gene Zif268 (also termed EGR-1, NGFI-A, and Krox-24), which is rapidly and transiently expressed in response to neuronal activation. The GAD67 promoter region contains a conserved Zif268 binding site (
15,
16), and Zif268 activation is accompanied by increased GAD67 expression in rat hippocampal neurons (
17). In contrast to other immediate early genes, Zif268 is expressed at stable, relatively high basal levels in the brain, and the expression of Zif268 is layer-specific in the cortex (
18–
23). Together, these data suggest that Zif268 mRNA expression in certain neuronal populations may play an important role in maintaining baseline cortical physiology while also regulating gene expression in response to particular stimuli. Given reports of lower levels of Zif268 mRNA in the dorsolateral prefrontal cortex from small samples of subjects with schizophrenia (
24,
25), reduced cortical Zif268 expression in schizophrenia may be responsible for lower GAD67 mRNA levels in the illness. However, neither the relation between Zif268 and GAD67 expression nor the molecular and cellular specificity of altered Zif268 mRNA expression has been directly examined in schizophrenia. For example, since immediate early genes are generally expressed in an activity-dependent manner (
26), it is important to determine whether changes in Zif268 mRNA expression differ from those of other immediate early genes in the dorsolateral prefrontal cortex of subjects with schizophrenia.
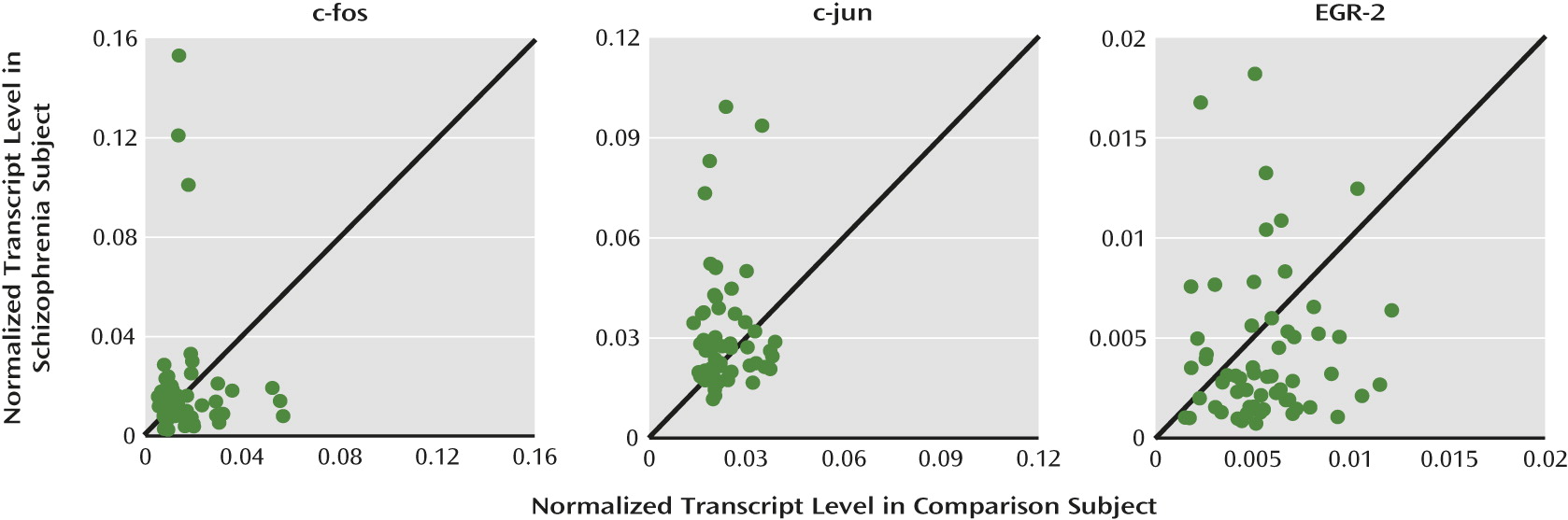
To address these questions, we used quantitative polymerase chain reaction (qPCR) and in situ hybridization to determine expression levels of Zif268 mRNA in the dorsolateral prefrontal cortex from a large cohort of schizophrenia and matched nonpsychiatric comparison subjects, the within-subject relationship between Zif268 and GAD67 mRNA levels, and the effects of potentially confounding variables. To determine the specificity of the relationship between Zif268 and GAD67 mRNA levels, we also quantified the expression of other regulatory immediate early genes (c-fos, c-jun, and EGR-2).
Discussion
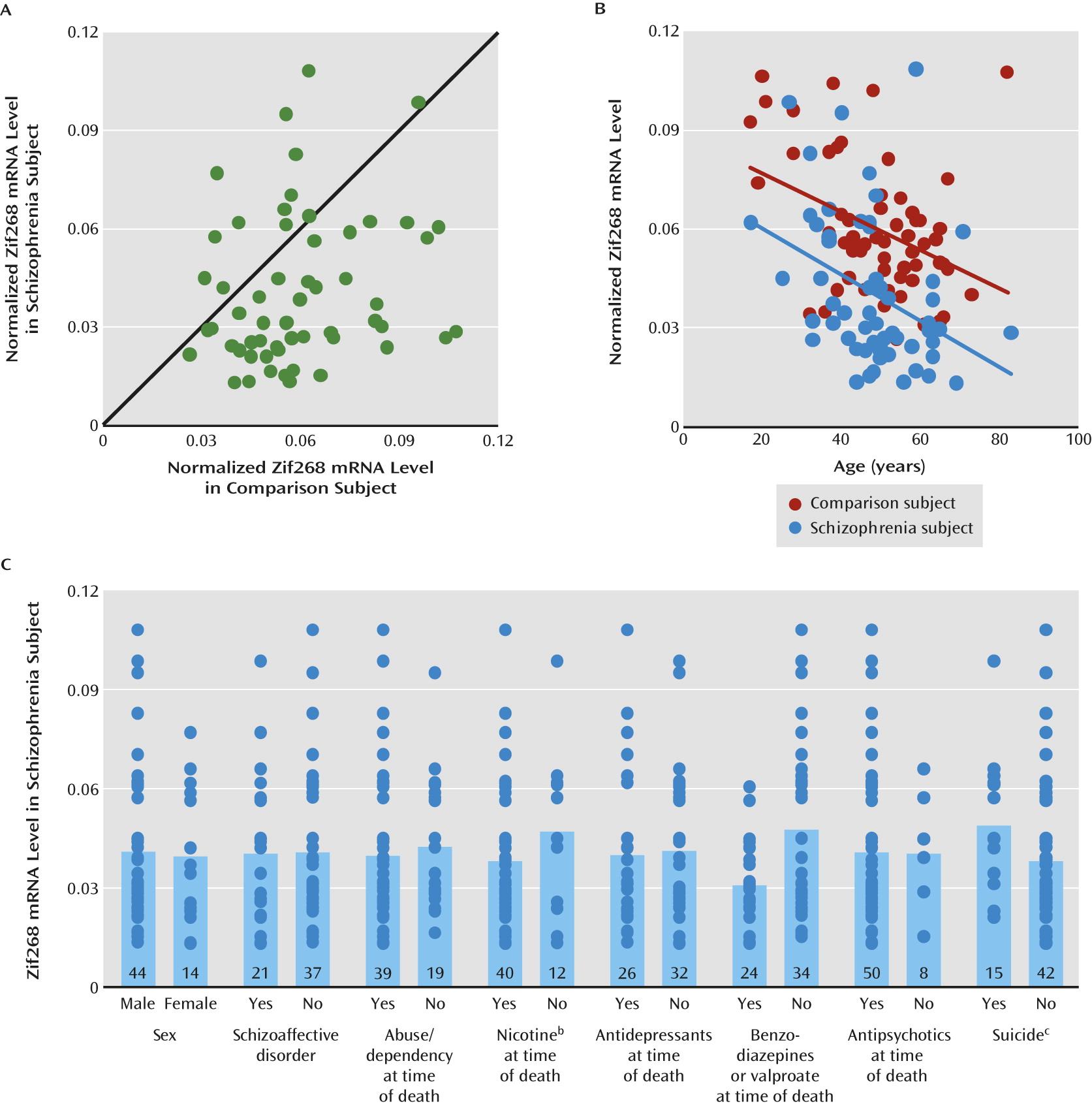
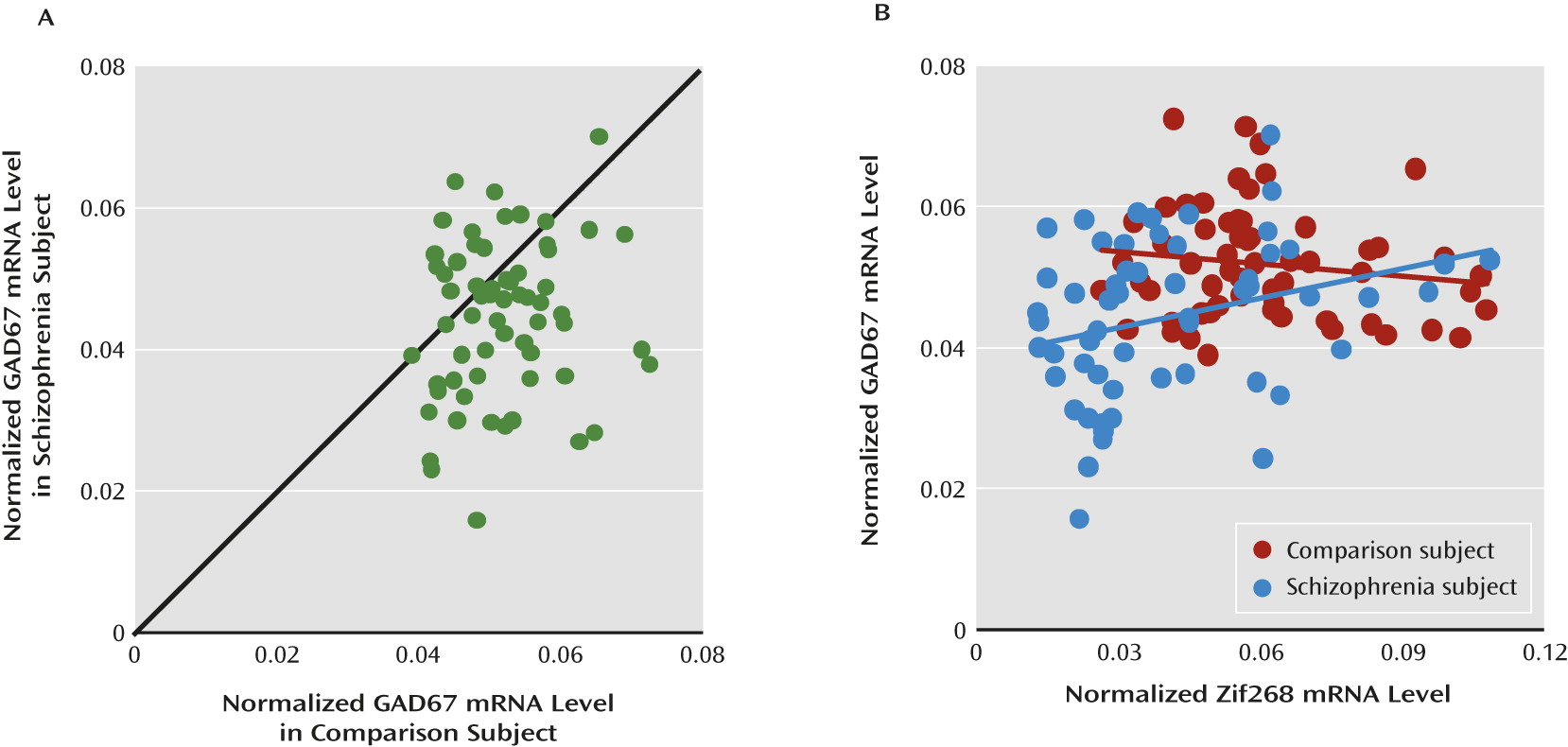
Lower levels of GAD67 mRNA and protein have been consistently found in the dorsolateral prefrontal cortex of subjects with schizophrenia (
2–
10). In this study, we focused on whether altered expression of the transcriptional regulatory factor Zif268 might contribute to lower cortical GAD67 levels in schizophrenia subjects. Consistent with the results of previous studies in small cohorts (
24,
25), we found that Zif268 mRNA levels were significantly lower in the dorsolateral prefrontal cortex of schizophrenia subjects. Furthermore, we replicated previous findings of lower GAD67 mRNA levels (
9) in a new cohort of schizophrenia subjects. Because Zif268 binds to the promoter region of the
GAD1 gene and can regulate GAD67 expression (
17), we hypothesized that deficient expression of Zif268 might be “upstream” of lower GAD67 mRNA expression in the dorsolateral prefrontal cortex of schizophrenia subjects. Consistent with this hypothesis, the expression levels of Zif268 and GAD67 mRNAs were positively correlated in the schizophrenia subjects. In addition, mRNA levels of other representative regulatory immediate early genes were not correlated with GAD67 mRNA levels in either schizophrenia or comparison subjects, suggesting that lower expression of Zif268 mRNA might be specific in schizophrenia, and thus contribute to lower cortical GAD67 levels in the disorder. However, the present study cannot exclude the possibility that lower GAD67 mRNA expression in schizophrenia is also regulated by other mechanisms. For example, allelic variants in the
GAD1 gene that are associated with risk for schizophrenia (
32) and altered histone methylation of the promoter regions of
GAD1 (
33) both appear to affect GAD67 mRNA levels in the dorsolateral prefrontal cortex, suggesting that multiple factors may contribute to lower cortical GAD67 levels in schizophrenia. Alternatively, since not all schizophrenia subjects showed lower Zif268 or GAD67 expression, lower levels of Zif268 mRNA may drive altered GAD67 expression in only a subset of individuals with schizophrenia (
27).
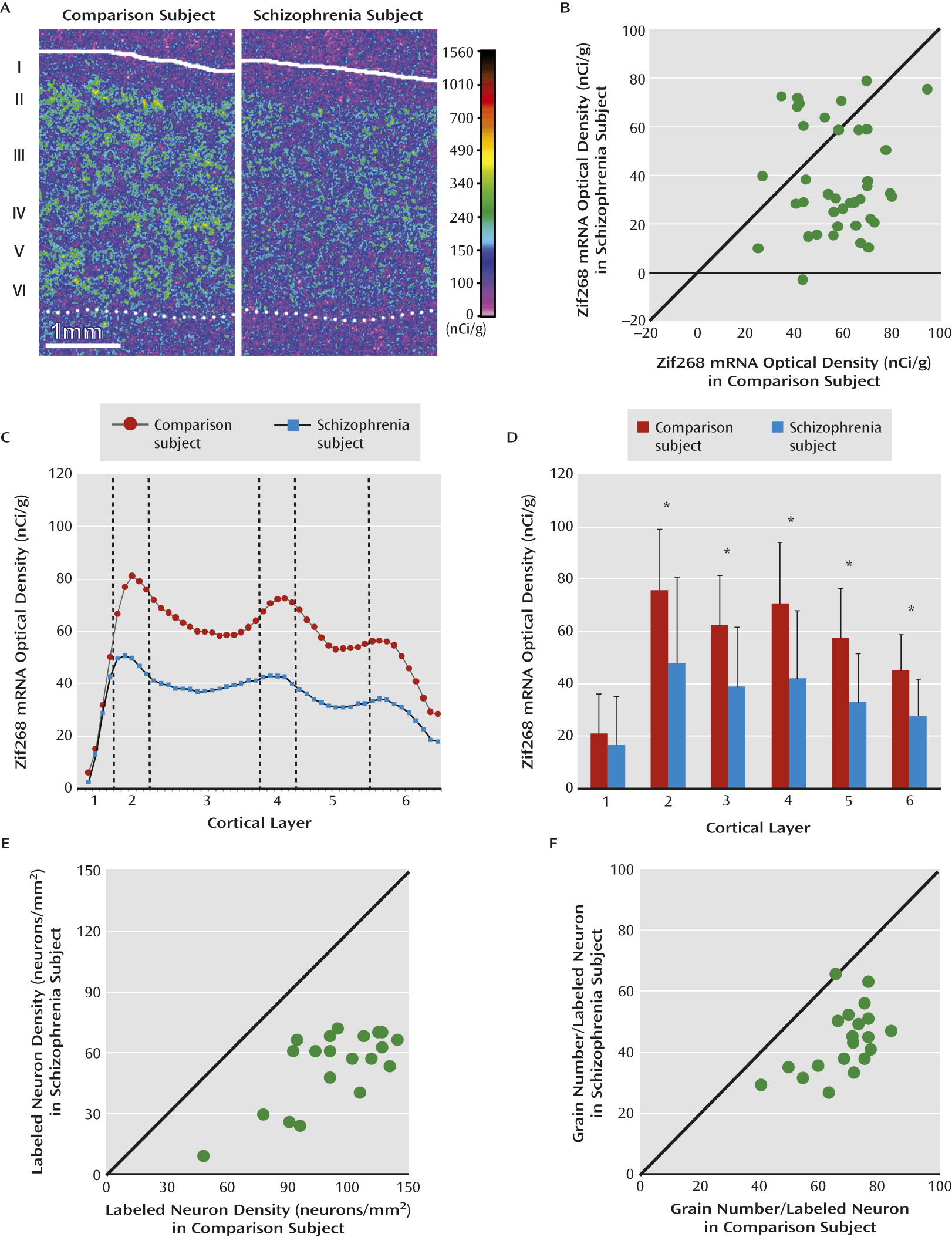
Our in situ hybridization study revealed a distinctive laminar pattern of Zif268 expression in human dorsolateral prefrontal cortex similar to that reported for the rodent, cat, and monkey cortex (
18,
19,
21–
23). Specifically, Zif268 mRNA-positive neurons were distributed across layers 2–6, with the highest densities in layers 2 and 4. Consistent with the qPCR findings, Zif268 mRNA levels were significantly lower in layers 2–6 in schizophrenia subjects relative to comparison subjects. At the cellular level, we found that a subset of neurons failed to express detectable levels of Zif268 mRNA and that other neurons expressed lower Zif268 mRNA levels in the dorsolateral prefrontal cortex of schizophrenia subjects. Together, the lower levels of Zif268 mRNA, which were demonstrated at different levels of resolution using two different methods, support the interpretation that Zif268 mRNA levels are lower in the dorsolateral prefrontal cortex of schizophrenia subjects and thus may contribute to lower expression of GAD67.
Although other classes of GABA neurons may be affected, only the subset of GABA neurons containing the calcium-binding protein parvalbumin have been directly shown to have lower GAD67 mRNA expression in schizophrenia (
30). Recently, we successfully performed microarray analyses from individually dissected neurons labeled with
Vicia villosa agglutinin, which is present in the perineuronal nets that surround parvalbumin neurons (
29). In the healthy comparison subjects used in the present study, Zif268 mRNA was highly expressed in the labeled neurons. This level of expression was similar to that of parvalbumin and was much higher than that of any other regulatory immediate early genes. In the labeled neurons, the mean level of Zif268 mRNA was 16% lower in the schizophrenia subjects relative to the comparison subjects, although this difference did not achieve statistical significance. The smaller magnitude of this difference relative to that observed by in situ hybridization may reflect the fact that only parvalbumin neurons with relatively well preserved perineuronal nets were sampled in the schizophrenia subjects; that is, the density of perineuronal nets identified by
Vicia villosa agglutinin labeling is lower in schizophrenia (
34,
35), suggesting that the most severely affected parvalbumin neurons were not captured by laser microdissection (
29). Furthermore, for the 14 subject pairs used in the microarray study, our qPCR study of gray matter found that Zif268 mRNA levels were only 22% lower in the schizophrenia subjects, compared with the 34% deficit observed in the remaining 48 schizophrenia subjects of the entire cohort of 62 subject pairs. These data indicate that Zif268 mRNA is heavily expressed in the parvalbumin population of GABA neurons and suggest that lower Zif268 mRNA expression in those neurons may contribute to lower GAD67 mRNA levels in schizophrenia.
Several lines of evidence indicate that the lower Zif268 mRNA expression is due to the disease process of schizophrenia and is not attributable to potential confounding factors frequently associated with the illness. First, none of the potentially confounding clinical factors, such as sex, diagnosis of schizoaffective disorder, history of substance dependence or abuse, nicotine use at the time of death, use of antipsychotics or antidepressants at the time of death, or death by suicide, accounted for lower Zif268 mRNA expression in the schizophrenia subjects. Although the use of benzodiazepines or sodium valproate at the time of death was associated with lower Zif268 mRNA expression, schizophrenia subjects who had not taken these medications still had significantly lower Zif268 mRNA levels than their matched comparison subjects. Second, Zif268 mRNA expression did not differ in the dorsolateral prefrontal cortex of monkeys chronically exposed to either haloperidol or olanzapine. Finally, a significant negative correlation between age and Zif268 mRNA level was seen in both schizophrenia and comparison subjects. The regression line for schizophrenia subjects was parallel to and shifted downward from that of comparison subjects, indicating that lower expression of Zif268 mRNA in schizophrenia subjects is present across adult life and thus is unlikely to be a consequence of illness chronicity. However, we cannot exclude the possibility that Zif268 mRNA expression declines during the early stages of clinical illness after onset of psychosis. Furthermore, this observation suggests that the deficient expression of Zif268 mRNA might be present early in the course of the illness and thus could contribute to the pathophysiology of the clinical features of the illness that reflect altered GABA neurotransmission.
Since all immediate early genes are activity dependent, it is possible that lower Zif268 mRNA levels in schizophrenia simply reflect a nonspecific consequence of reduced cortical neuronal activity associated with prefrontal cortex dysfunction (“hypofrontality”). However, this interpretation seems unlikely since 1) lower Zif268 mRNA levels were not related to illness chronicity or other factors that frequently accompany schizophrenia and 2) mRNA levels of other regulatory immediate early genes examined in the present study were not lower in the schizophrenia subjects. In fact, the other three immediate early genes examined were lower, unchanged, or higher in expression in schizophrenia subjects. Consistent with these results, accumulating evidence suggests that the expression of each immediate early gene can be individually regulated by selective signaling pathways. For example, various stimulation paradigms that induce long-term potentiation result in increased mRNA levels of Zif268 but not of other members of the fos/jun family (
36,
37). Likewise, high-frequency electrical stimulation of the mediodorsal thalamic nucleus was found to result in increased expression of Zif268 mRNA, but not of c-fos mRNA, in rat frontal cortex (
38). Taken together, these findings suggest that the Zif268 signaling pathway may be selectively altered in schizophrenia, and thus contribute to lower cortical GAD67 levels in the disorder.
Of course, the factors leading to lower Zif268 mRNA expression in a majority of schizophrenia subjects remain to be determined. One possibility could be reduced activity in specific circuits of the dorsolateral prefrontal cortex, such as those furnished by layer 3 pyramidal cells (
14). These cells have a lower density of dendritic spines (
39,
40), the principal site of excitatory synaptic inputs, and thus they presumably receive less excitatory drive from other cortical regions or the thalamus (
41). The axons of dorsolateral prefrontal cortex layer 3 pyramidal cells project across all cortical layers (
42), and thus reduced activity in these cells could account for the observed laminar distribution of lower Zif268 mRNA expression in subjects with schizophrenia.
In summary, the findings of this study suggest that lower levels of Zif268 mRNA may be one upstream molecular factor leading to lower GAD67 mRNA levels in schizophrenia, providing a potential mechanistic basis for impaired GABA synthesis in the illness.