Heightened amygdala reactivity to negative emotional stimuli is commonly observed in functional imaging studies of patients with major depressive disorder (
1,
2). However, the extent to which such amygdala reactivity is a premorbid risk factor for the emergence of syndromal depression remains unclear. Cross-sectional studies reporting increased amygdala reactivity in patients compared with controls cannot determine whether this finding reflects a premorbid risk factor or a pathophysiologic consequence of a disorder. Prospective evaluation of amygdala reactivity before a depressive disorder develops is thus necessary to determine whether this neural phenotype represents a premorbid risk factor.
A positive family history of psychopathology constitutes a robust and replicated individual risk factor for depression (
3–
5). Thus, a prospective neuroimaging study of individuals at differential familial risk for major depression represents a viable strategy for evaluating amygdala reactivity as a premorbid risk factor. Positioning such a prospective study during the transitional developmental window of adolescence, which marks the beginning of a period of heightened risk for the development of mood disorders (
6,
7), allows for assessment of neural markers prior to the emergence of a disorder but within a relatively short temporal frame that allows for mapping of these markers onto subsequent psychopathology.
To date, only a handful of studies have examined differences in neural function associated with familial risk (
8–
13), with several reporting heightened amygdala reactivity in individuals at elevated risk for depression. For instance, adolescents at familial risk for depression exhibit increased amygdala reactivity during sad mood induction (
8) and during viewing of fearful facial expressions (
9). However, sample sizes have been small, age ranges have been wide, there has not been explicit consideration of current depressive symptoms, and given the cross-sectional nature of these studies, it is still unknown how this potential neural risk marker emerges.
Identifying the developmental emergence of neural phenotypes associated with risk can inform efforts toward early risk detection and intervention. Research on structural brain development suggests that developmental trajectories may be more predictive of outcomes than a single measurement at one time point (
14). Moreover, as Casey and colleagues (
15) proposed, developmental trajectories can serve as endophenotypes when examining the influence of genetic risk on psychopathology. Identifying the timing and nature of atypical development of amygdala reactivity will also be critical in advancing our ability to predict, and ultimately prevent, the emergence of clinical disorders. Nevertheless, the longitudinal development of amygdala reactivity has not been characterized in a high-risk cohort.
One hypothesis suggests that the heightened amygdala reactivity associated with depression and other internalizing disorders may emerge as a result of altered trajectories of neural development during adolescence (
16). Specifically, cross-sectional studies indicate that typical development in adolescence entails a decrease in amygdala reactivity to emotional facial expressions with age (
17,
18). These findings led us to predict that adolescents at familial risk for depression would fail to exhibit this typical decline in reactivity.
Although family history of depression is a robust predictor of risk for a depressive disorder, many individuals without a family history will develop depression. For these individuals, environmental stressors such as childhood maltreatment and stressful life events appear to play a critical role in increasing vulnerability for the disorder (
19–
21). Childhood maltreatment and adolescent life events are associated with heightened amygdala reactivity to negative stimuli (
22,
23), suggesting that these risk factors may affect amygdala development. Therefore, we also examined the effect of these risk factors on the development of amygdala function.
While there is evidence for main effects of family history and life stress, no research has yet examined the interaction of these risk factors on amygdala reactivity. On the one hand, some evidence indicates that life stress may increase vulnerability in individuals with a family history of depression (
24). On the other hand, high levels of life stress may not be necessary for individuals with a positive family history to develop depression. Indeed, individuals who develop depression but have a negative family history are more likely than those with a positive family history to report experiencing a life event prior to the onset of depression (
25). Likewise, unaffected adult relatives with a positive family history of depression report higher levels of depressive symptoms than individuals with a negative family history, but this association is not moderated by early life stress (
26). Thus, individuals with a family history of depression may evidence altered amygdala development even if they have experienced relatively low levels of life stress.
In this study, we examined the development of amygdala reactivity in adolescents at high or low risk for depression as a function of family history of depression, and the moderating effects of stress. Participants were on average 13 and 15 years old at the first and second waves, respectively. Given that the peak period of onset for major depression in individuals with a positive family history is between ages 15 and 20 (
4,
5), we expected to observe differences in amygdala reactivity before the emergence of a disorder, in line with our hypothesis that increased amygdala reactivity is a premorbid neural biomarker of risk. Based on the developmental model proposed above, the following hypotheses were examined. First, threat-related amygdala reactivity will vary as a function of familial risk, with low-risk adolescents (those from families with no history of depression) exhibiting decreases in reactivity with age and high-risk adolescents (those with a positive family history) exhibiting no change or an increase in reactivity with age. Second, individuals with higher levels of childhood and adolescent life stress will also fail to show declines in amygdala reactivity with age, and these effects may differ based on presence or absence of a family history of depression. To confirm that differences in amygdala reactivity are premorbid, we examined these hypotheses in our full sample and in a subsample that excluded participants who were diagnosed with an internalizing disorder subsequent to their enrollment in the study.
Method
Participants
Participants were recruited as part of the Teen Alcohol Outcomes Study (TAOS), the aim of which is to examine the association between the development of depression and alcohol use disorders in adolescence. Sampling and recruitment procedures for the TAOS have been described in detail previously (
27,
28). After receiving a complete description of the study, parents provided written informed consent and participants provided assent, following procedures approved by the Institutional Review Board at the University of Texas Health Sciences Center San Antonio. Participants were 11–15 years old during the first wave of data collection. Inclusion in the high-risk group required the presence of a first- and a second-degree relative with a history of major depression. Inclusion in the low-risk group required that the participant have no first- or second-degree relatives with a history of depression. Participants also had to be free of psychiatric diagnoses at the baseline assessment, with the exception that an anxiety diagnosis was permitted in the high-risk group. Diagnoses were assessed through structured clinical interviews with the adolescent and parent separately, using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (
29). Participants were also excluded if they met criteria for a substance use disorder or reported any binge drinking at baseline (based on National Institute on Alcohol Abuse and Alcoholism guidelines).
Participants were recontacted annually for diagnostic interviews and questionnaire measures, and they underwent follow-up functional MRI (fMRI) scanning during the third wave of data collection, although a small proportion (14%) completed the second scan at the fourth wave. The mean interval between the first and second scans was 2.07 years (SD=0.40). Attrition and exclusion of participants for quality control are reported in the
data supplement that accompanies the online edition of this article. The final sample consisted of 232 participants (120 and 112 in the high- and low-risk groups, respectively) at the baseline scan and 197 participants (101 and 96 in the high- and low-risk groups, respectively) at the second scan (
Table 1; see also Figure S1 in the
data supplement). Usable fMRI data from both the first and second scans were available for 157 of these participants (85 and 72 in the high- and low-risk groups, respectively).
In the final sample, approximately 57% of participants were white/Caucasian, 39% were Hispanic, 3% were African American, and 1% were Asian or American Indian. There was no significant difference in the racial/ethnic distribution between the high- and low-risk groups. There was no significant difference between groups in gender ratio (51% of participants in the high-risk group were female, and 49% in the low-risk group) or in handedness (9% of participants in the high-risk group were left-handed, and 10% in the low-risk group). A portion of the participants were siblings (11 pairs in the high-risk group, 13 in the low-risk group). Thirty-two participants in the high-risk group had an anxiety diagnosis at baseline (see the online data supplement). Sixteen participants (15 of them in the high-risk group) developed major depressive disorder and three (two of them in the high-risk group) developed an anxiety disorder before the second scan.
Procedure
fMRI paradigm.
Participants performed an emotional face matching task that has been shown to reliably elicit amygdala reactivity in numerous studies of adults, as well as in the baseline scan of the present sample (
27,
28). Task blocks consisted of matching angry and fearful facial expressions, and control blocks consisted of matching geometric shapes. Further details of the task are presented in the online
data supplement.
Depressive symptoms.
Depressive symptoms were assessed with the child-report version of the Mood and Feelings Questionnaire (
30).
Childhood maltreatment.
Childhood maltreatment was assessed using the Childhood Trauma Questionnaire (
31). In line with previous research in this sample (
27,
28), the emotional neglect subscale was selected because of its greater variability.
Stressful life events.
Stressful life events occurring during the year preceding each scan were assessed using the Stressful Life Events Schedule (
32). This instrument was designed specifically to assess stressful life events in children and adolescents, and it probes for life events that are relevant to this age group. Each event is given a subjective rating of threat by the participant, as well as an objective rating by independent raters. Objective severity ratings for all events occurring within 12 months of scanning were then squared and summed, and this summed value was divided by the number of events reported, yielding a measure of life-event severity that was more heavily weighted by severe events (see the online
data supplement for additional details). In order to reduce the number of comparisons performed, objectively rated life stress was used (correlations with subjective life stress at each wave were ∼0.5), based on stronger evidence for effects of stressful life events on depression when objective measures are used (
33).
Analysis of fMRI Data
Blood-oxygen-level-dependent (BOLD) fMRI data were analyzed using SPM8 (
http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Images from the first and second scans were then entered into second-level models, and a conjunction analysis was performed to extract BOLD parameter estimates from functional clusters that were activated across both scanning sessions within the left or right amygdala at p<0.05, family-wise error corrected. Further details on quality control procedures and extraction of amygdala reactivity values are provided in the online
data supplement.
Hypothesis 1: Developmental Change in Amygdala Reactivity as a Function of Family History of Depression
After extracting mean parameter estimates of amygdala reactivity in SPM8, all subsequent analyses were performed in SPSS, version 21 (IBM, Armonk, N.Y.). To examine whether the association between amygdala reactivity and age differed between the groups, linear mixed models were applied following the procedures of previous longitudinal neuroimaging research (
34,
35). Using the methods specified by Garson (
36), a three-level linear mixed model was constructed in SPSS with wave (level 1) nested within participant (level 2) nested within family (level 3). A risk group-by-age interaction was evaluated to test whether change in amygdala reactivity with age was moderated by risk group. We also reran analyses excluding any participants with an internalizing disorder and controlling for depressive symptoms at each wave. Further details about the modeling procedure are provided in the online
data supplement.
Hypothesis 2: Developmental Change in Amygdala Reactivity as a Function of Life Stress
Linear mixed models were used to examine the effect of childhood and adolescent stress on change in amygdala reactivity with age. Centered scores on the emotional neglect subscale of the Childhood Trauma Questionnaire and the Stressful Life Events Schedule for wave 1 and the Stressful Life Events Schedule for wave 2 were simultaneously entered as covariates in a linear mixed model with amygdala reactivity as the dependent measure. Main effects and interactions with age and risk group were also entered, and gender was included as a covariate.
Results
Hypothesis 1: Developmental Change in Amygdala Reactivity as a Function of Family History of Depression
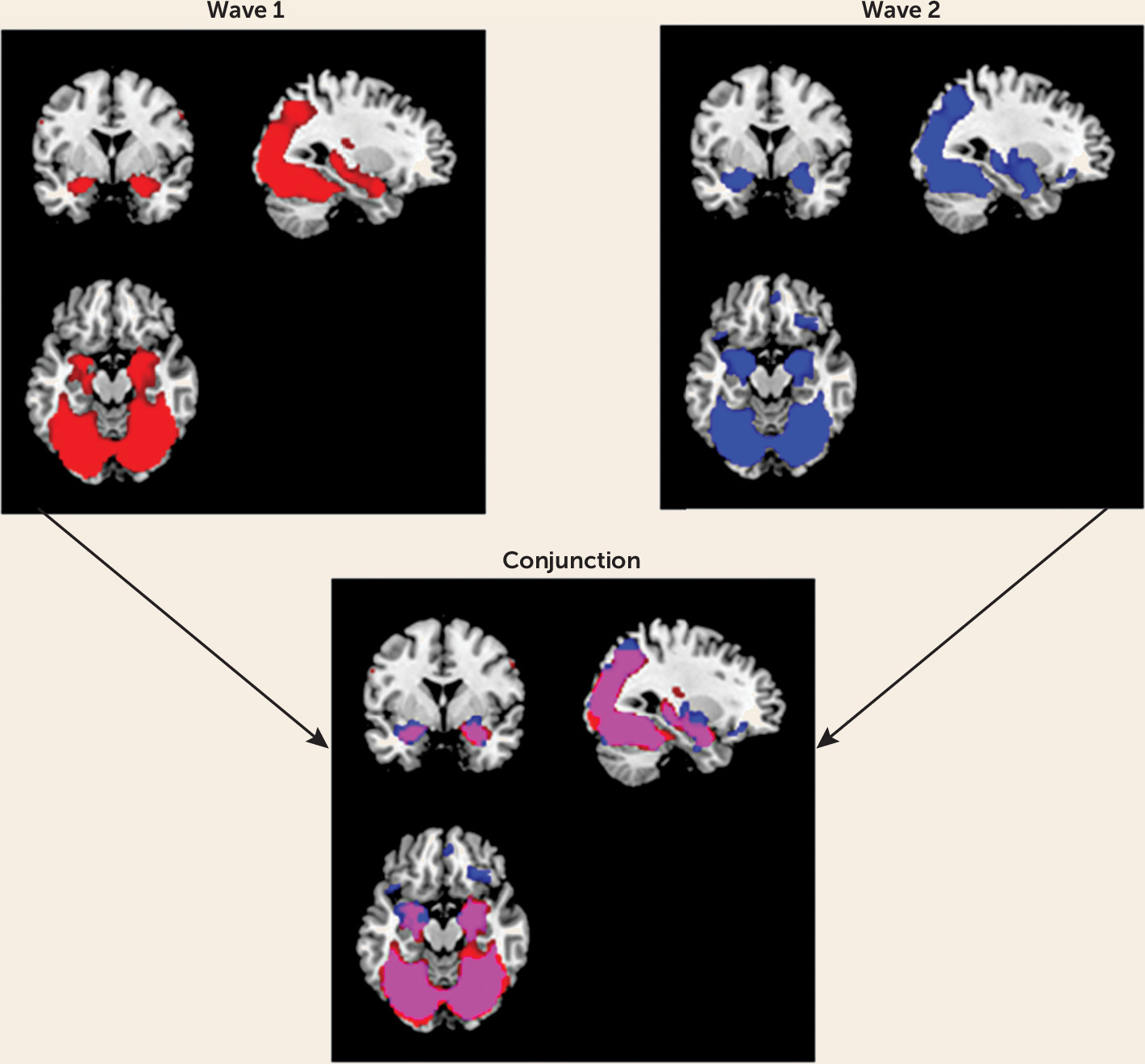
First, the main effect of task was examined in SPM8 to ensure that amygdala reactivity was elicited. Mean whole-brain activation maps for the main effects of task contrast (i.e., all faces > geometric shapes) were visually inspected. As seen in the overlap between the two activation maps (
Figure 1), the task reliably elicited reactivity in expected corticolimbic regions to a similar spatial extent at both waves. More specifically, the task produced robust bilateral amygdala reactivity at both waves (wave 1: left amygdala, t=15.75, df=231, p<0.001; peak coordinates, −22, −2, −18; right amygdala, t=19.03, df=231, p<0.001; peak coordinates, 22, −4, −18; wave 2: left amygdala, t=17.83, df=196, p<0.001; peak coordinates, −20, −4, −16; right amygdala, t=18.38, df=196, p<0.001; peak coordinates, 20, −4, −16).
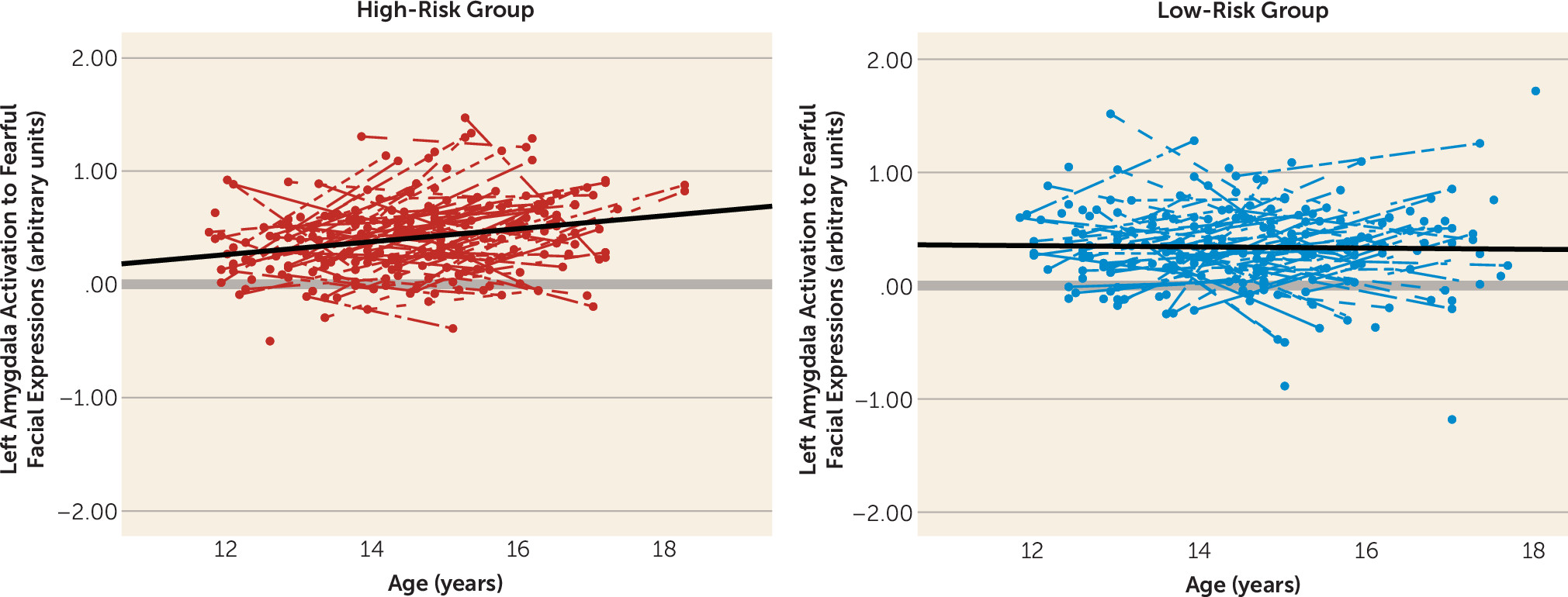
Next, we examined the risk group-by-age interaction using linear mixed models in SPSS to test whether the groups differed in changes in amygdala reactivity with age. This model provided a significantly better fit to the data relative to the null model (χ
2=21.13, df=5, N=427, p<0.001). There was a risk group-by-age interaction for left amygdala reactivity to fearful faces, which survived Bonferroni correction for multiple comparisons (F=6.67, df=1, 220, p=0.010) (
Figure 2). This was driven by a significant increase in amygdala reactivity in the high-risk group (p<0.001), whereas the low-risk group remained stable with age. The effects of age, risk group, and their interaction explained an additional 9% of variance (7% for main effects and 2% for their interaction). This interaction remained significant when we controlled for mean head displacement, mean accuracy, and mean reaction time on the task (F=6.37, df=1, 217, p=0.012). This effect also remained significant when we excluded participants with an internalizing diagnosis and controlled for depressive symptoms (F=5.29, df=1, 131, p=0.023). Effects of gender or a quadratic effect of age were not significant and did not improve model fit. There was a similar pattern for right amygdala reactivity to fearful faces, although the interaction was not significant (see Figure S2 in the online
data supplement). Results were specific to fearful faces, as the interactions for angry faces were not significant. Thus, only left amygdala reactivity to fearful facial expressions was used in subsequent analyses to reduce the number of comparisons performed.
Hypothesis 2: Developmental Change in Amygdala Reactivity as a Function of Life Stress
The overall test of model fit including childhood trauma and life stress at both waves was significant, with the interaction between risk group and stressful life event severity assessed at wave 1 having an effect on amygdala reactivity (F=8.25, df=1, 180, p=0.005). Given that the test of model fit penalizes for including parameters that do not predict the dependent variable, we removed the effects of childhood trauma and stressful life events at wave 2 from the model. The simplified version of the model was a significantly better fit relative to the null model (χ
2=35.18, df=12, N=415, p<0.001). Within this model, three interaction terms were significant predictors of the change in left amygdala reactivity to fearful faces: the interaction of risk group by age (F=6.41, df=1, 185, p=0.012), the interaction of risk group by stressful life event severity assessed at wave 1 (F=6.87, df=1, 42, p=0.012), and the interaction of age by stressful life event severity (F=4.27, df=1, 151, p=0.04). Adding the effects of life stress accounted for an additional 2% of variance, for a total of 11% of variance explained. These effects remained significant when we controlled for accuracy, reaction time, and head displacement. These interactions were also significant when we excluded participants with an internalizing disorder and controlled for depressive symptoms (risk group by age, F=5.54, df=1, 104, p=0.021; risk group by stress, F=8.05, df=1, 51, p=0.007; age by stress, F=9.29, df=1, 86, p=0.003). To interpret these effects, predicted outcomes for amygdala reactivity were estimated as a function of the parameters in the model, as described by Preacher et al. (
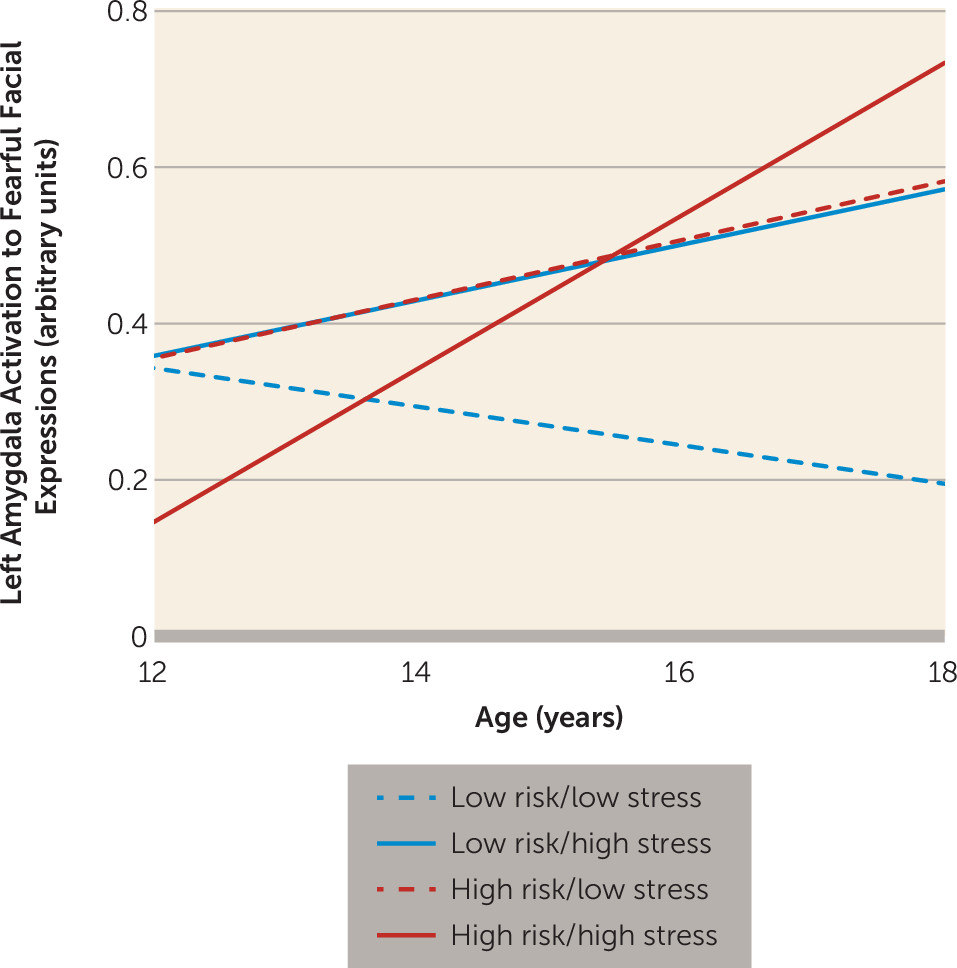
37). As illustrated in
Figure 3, participants in the high-risk group exhibited increases in amygdala reactivity with age, regardless of the amount of life stress experienced in early adolescence. In contrast, low-risk adolescents exhibited the expected pattern of decreasing amygdala reactivity with age under conditions of low stress; however, under higher levels of stress, low-risk adolescents exhibited increases in reactivity with age.
Discussion
Our aim in this study was to address two gaps in our knowledge of whether and how variability in threat-related amygdala reactivity contributes to the development of major depression. The first was whether heightened amygdala reactivity is a premorbid neural risk marker for depression, observable in at-risk individuals before the onset of clinical symptoms or disorder. The second was how and when this neural phenotype emerged. Our results work toward filling these gaps by demonstrating that heightened amygdala reactivity is observed prior to the emergence of clinical symptoms or disorder and represents a divergence of developmental trajectories over the course of adolescence.
Our results reveal that positive family history of depression and stressful life event severity affect the development of amygdala reactivity during adolescence. Adolescents with a positive family history of depression consistently evidenced a pattern of increasing amygdala reactivity with age, even if they experienced relatively mild life stress in early adolescence. Adolescents with a negative family history of depression evidenced decreases in amygdala reactivity with age under low stress, in line with our predictions based on cross-sectional work. However, adolescents in the low-risk group who experienced relatively high levels of life stress in early adolescence evidenced increases in amygdala reactivity with age, similar to the high-risk group. This interaction suggests that two distinct risk factors—family history of depression and life stress—may influence risk for depression by a common developmental pathway of altered development of amygdala reactivity during adolescence. These findings also fit within the framework of allostatic load, which posits that chronic dysregulation of the stress response induces structural and functional changes in the brain, including the amygdala (
38). Dysregulation of the stress response (either through a combination of genetic and environmental risk factors in the high-risk group or through severe life stress in the low-risk group) could therefore lead, over time, to long-term changes in amygdala reactivity, as demonstrated here. Our results also suggest that group differences in amygdala reactivity are minimal in early adolescence and grow stronger with age (
Figures 2 and
3). This could partially reflect the fact that we excluded participants with psychiatric disorders at baseline; however, additional research is needed to investigate whether stress-related changes in amygdala reactivity are accelerated by developmental factors in adolescence (e.g., pubertal developmental, the transition to high school).
In line with our conceptualization of increased amygdala reactivity as a premorbid biomarker of risk, differences in reactivity were evident in participants who had not yet developed internalizing disorders, as well as when we controlled for depressive symptoms. Individuals at familial risk for depression are most likely to develop the disorder in late adolescence and young adulthood, with a peak period of onset between ages 15 and 20 (
4,
5). Therefore, the changes in amygdala reactivity observed in early to mid adolescence may increase vulnerability for the disorder later in development. The TAOS is an ongoing longitudinal study, and data continue to be collected. We plan to further evaluate the predictive utility of our observed differences in the developmental trajectories of amygdala reactivity on the emergence of clinical depression as the cohort is followed into late adolescence and early adulthood. If it is possible to detect heightened risk for depression through neuroimaging measures before dysfunction becomes apparent through self-report of symptoms, this could be of clinical utility in the early detection of risk.
Our study has several limitations. First, the fMRI paradigm we used included only fearful and angry facial expressions; it is unclear whether similar results would be observed for other negative or positive emotional stimuli (e.g., sad or happy facial expressions) that have previously been implicated in risk for depression (
8,
9,
12). Second, most participants reported experiencing relatively mild levels of stress as assessed by the Childhood Trauma Questionnaire and the Stressful Life Events Schedule. Indeed, the low levels of emotional neglect reported on the Childhood Trauma Questionnaire may explain the lack of a significant effect on amygdala development. Finally, while we identified effects explaining 11% of the total variance in amygdala reactivity, identifying additional genetic, epigenetic, and environmental moderators of these effects (
27,
28,
39,
40) may further help account for individual differences in amygdala reactivity over the course of development.
These limitations notwithstanding, our prospective design, stratification based on familial risk, repeated neuroimaging with a reliable probe of amygdala reactivity, and large sample size all represent major strengths. Additional research with this sample will be needed to determine whether the altered pattern of amygdala development observed here predicts the onset of clinical depression during later adolescence and young adulthood. If so, this pattern of development could inform efforts toward early detection of risk for the development of depression and possibly other psychopathology.