Posttraumatic stress disorder (PTSD) is an anxiety disorder characterized by avoidance, re-experiencing, and hyperarousal symptoms following exposure to a traumatic life event. However, a diagnosis of PTSD has been linked to high rates of illness (
1) and inflammatory disease (
2), including cardiovascular (
3) and metabolic disease (
4). Individuals with PTSD show elevated levels of the inflammatory cytokines interleukin-6 (IL-6), IL-1β, and IL-2 (
5–
7), augmented nuclear factor-κB (NF-κB) gene expression (
8) and activity (
9), and altered immune cell sensitivity to glucocorticoids (
10). Furthermore, peripheral levels of inflammatory molecules correlate with PTSD ratings (
11). These data linking PTSD to a proinflammatory state further support the notion that the disorder is associated with chronic inflammation, in a manner similar to depression (
12).
More recently, increased levels of the proinflammatory marker C-reactive protein (CRP) have been described in individuals with PTSD (
13,
14). The directionality of the change in CRP levels in PTSD is equivocal, as other studies have shown decreased CRP levels in individuals with PTSD (
15) or even a lack of association between PTSD and CRP levels (
11,
16). However, a prospective study recently showed that increased baseline CRP prior to deployment was a significant predictor for PTSD, suggesting that increased inflammation is a risk factor for the disorder (
17). Genetic variations that cause an increase in CRP levels could also serve as a vulnerability factor for PTSD, as genetic traits significantly account for interindividual variability in CRP level (
18). Single-nucleotide polymorphisms (SNPs) are present in the
CRP gene that influence CRP level (
19) and confer increased individual vulnerability to cardiovascular disease (
20).
In the present study, we assessed whether particular SNPs that tag the
CRP gene at moderate levels of linkage disequilibrium (rs3093068, rs1205, rs3093067, rs1130864, rs3093066, and rs3091244) are associated with higher serum CRP levels, higher PTSD symptom ratings, and a higher odds ratio for likelihood of PTSD diagnosis. Furthermore, we sought to determine whether increased CRP levels are associated with PTSD in an urban population with high levels of trauma and a high lifetime prevalence of PTSD (
21). We hypothesized that CRP level in highly traumatized individuals would be associated positively with PTSD symptom ratings. We also assessed whether CRP level would be associated with fear-potentiated startle, a robust psychophysiological phenotype that is increased in PTSD (
22). These data will elucidate whether
CRP genetic variance is associated with heightened inflammation indicated by CRP level as well as augmented PTSD symptoms in trauma-exposed individuals. Together, these data suggest a potential mechanism by which increased proinflammatory states may modulate neurophysiology and heighten PTSD symptoms.
Discussion
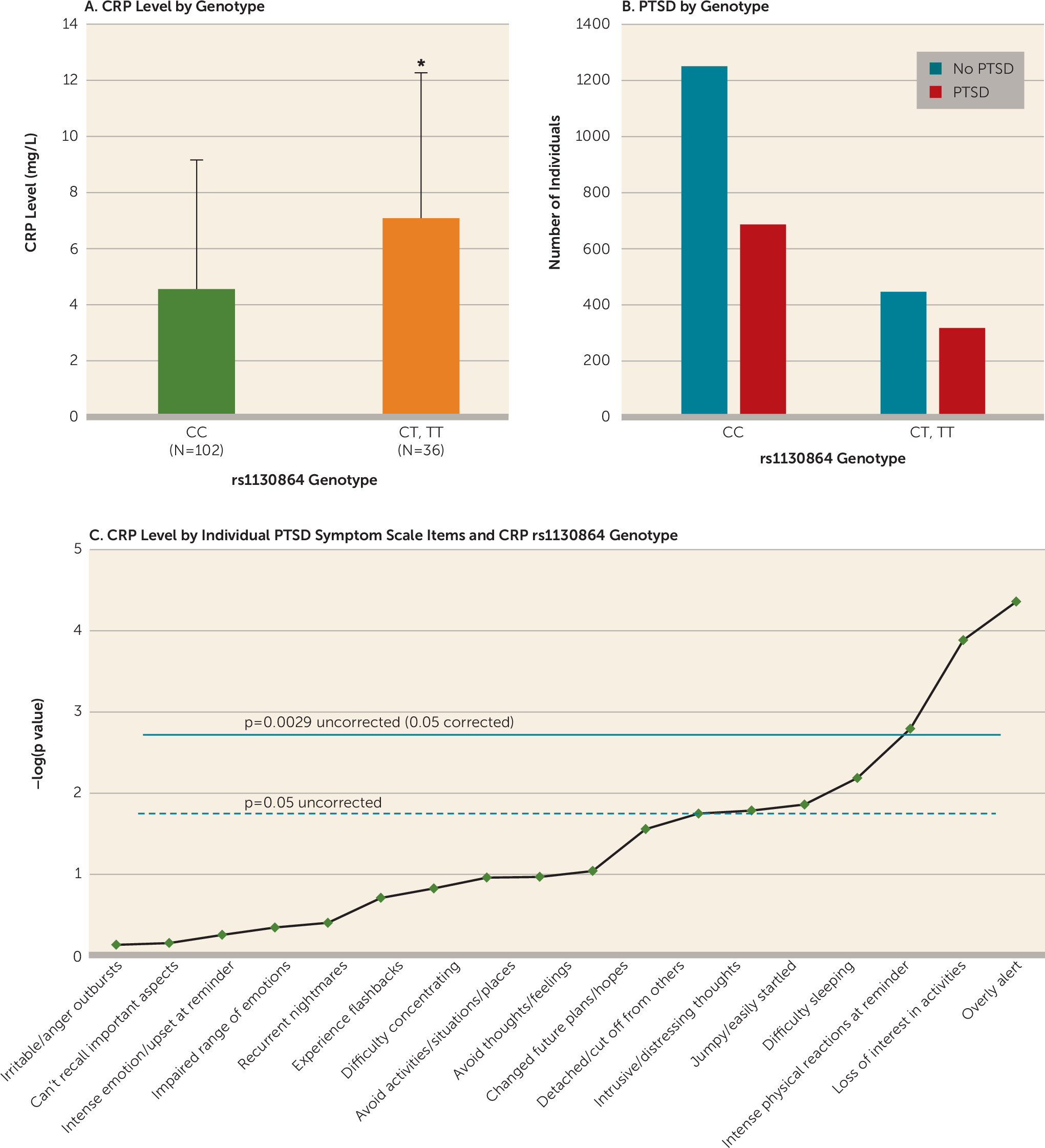
Our results indicate that genetic variation in the
CRP gene in traumatized individuals augments CRP levels and confers vulnerability to increased hypervigilance. While
CRP SNPs are linked to increased susceptibility to cardiovascular and metabolic disease and to increased CRP levels, this is the first demonstration that a SNP within the
CRP gene (rs1130864) is associated with PTSD symptoms and CRP levels in traumatized individuals. Furthermore, we demonstrated that elevated CRP levels are associated with increased psychophysiological hyperarousal as determined by increased fear-potentiated startle, exacerbated PTSD symptoms, and greater odds of a PTSD diagnosis. These observations are consistent with previous reports linking a proinflammatory state with psychopathology (
12), including PTSD (
5,
6,
9).
To our knowledge, this study is the first to describe a genetic locus within the
CRP gene—rs1130864—that is associated with increased susceptibility to a PTSD diagnosis and greater PTSD symptom levels. The rs1130864 SNP has previously been associated with increased CRP levels (
26), a result replicated here. While both rs1130864 and rs3091244 were the only two loci that were associated with CRP level, only rs1130864 survived multiple-test correction for effects on CRP level and PTSD symptoms. The data indicate that while the rates of PTSD were high in both rs1130864 allelic groups (CC and T) in our sample, paralleling the high overall rates of trauma in our inner-city population (
21), the differences between groups were largely based on three symptoms, highlighting the significant effects of the rs1130864 genotype on PTSD symptoms. Specifically, an analysis of the effects of rs1130864 on individual PTSD symptoms in trauma-exposed individuals yielded a strong association with being “overly alert,” suggesting that this genetic locus confers individual vulnerability to hypervigilance by influencing CRP level. Furthermore, a recent study (
33) suggested that PTSD in women and increased inflammation are correlated with intrusive re-experiencing, a hallmark symptom of PTSD. Increased alertness and re-experiencing in PTSD may in fact continually activate the stress axis and the immune system and thus dysregulate cytokine-glucocorticoid negative feedback (
34) and alter fear physiology (
35) in PTSD.
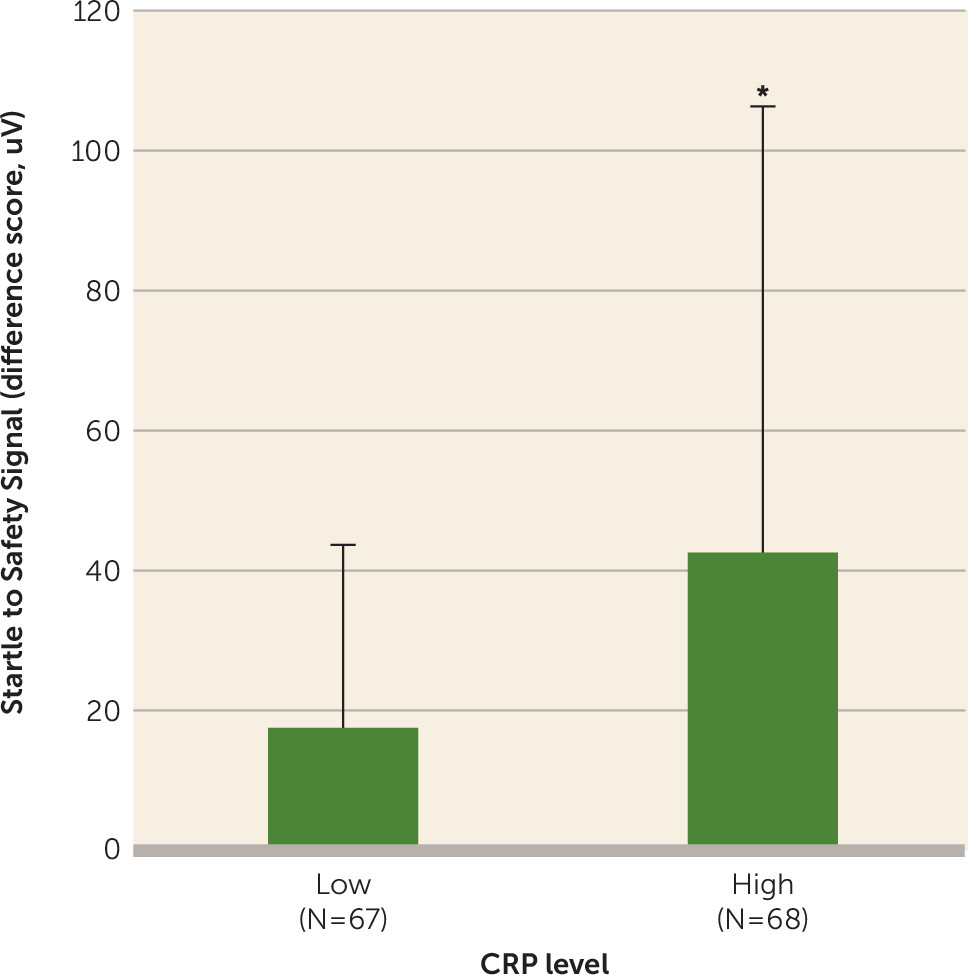
In the present study, higher CRP levels were associated with impaired inhibition of fear-potentiated startle to a safety signal, which is thought to be a biomarker for PTSD (
35) and is related to the severity of current PTSD symptoms (
36), and more specifically, hyperarousal symptoms (
22). This effect of CRP on fear physiology was independent of PTSD symptoms, indicating that inflammation may increase vulnerability to a heightened fear response. The association between CRP level and fear-potentiated startle was stronger in women than in men, supporting recent evidence for heightened fear-potentiated startle after puberty in females compared with males (
37) and the role of menstrual cycle phase in modulating fear-potentiated startle in females (
38). This sex difference in the CRP/fear-potentiated startle relationship also corroborates previous findings of higher vulnerability to PTSD in females (
39,
40) and data indicating sex differences in immune function that leave women more susceptible to increased inflammation (
41,
42). Further studies are necessary to elucidate the effects of increased inflammation and female gonadal hormones on fear psychophysiology.
The rs1130864 SNP that was associated with both CRP level and PTSD symptoms was not associated with fear-potentiated startle. The lack of a genotype effect on fear-potentiated startle should be considered preliminary, as it could be due to our small sample of participants who underwent fear-potentiated startle. While self-reported fear has been linked to increased CRP level in healthy individuals (
43), there have been no reports in humans or in rodents that describe the effects of inflammation on the psychophysiology of fear responses. It is important to note that the exaggerated neurobiological sensitivity to threat that is characteristic of anxiety disorders, including PTSD, can lead to increased activity of the stress and immune axes, and thus promotes a state of chronic inflammation (
44). These data, taken together with our finding that increased fear-potentiated startle is associated with greater serum CRP levels, provide a rationale for assessing the role of inflammation in fear responses and hypervigilance in future research.
Our results corroborate previous reports of heightened CRP levels in individuals with PTSD (
13,
14), while other studies have shown decreased CRP levels in individuals with PTSD (
15) or even a lack of association between PTSD and CRP level (
11,
16). The inconsistencies between these reports may be related to small sample sizes (
16), distinct study populations (
45), the presence of uncontrolled confounders (
13), and the use of control groups with high rates of infection (
15). The positive association between CRP level and PTSD symptoms in the present study was described in a highly traumatized inner-city sample consisting primarily of African American participants (
21). This population has a higher prevalence of PTSD (
21) as well as an elevated risk for cardiovascular and metabolic disease (
4,
46), suggesting that increased inflammation in this population may serve as an underlying mechanism by which psychopathology and illness manifest together. Indeed, even though our study could not account for comorbid physical illness in the sample, CRP levels were high (mean=5.14 mg/L, SD=4.77), which suggests that some traumatized individuals recruited to our study from primary care settings also suffered from physical illness. Future studies from clinical populations should analyze both illness comorbidity and the potential confounder of obesity through measures such as body mass index. Additionally, although our study is the largest to date examining CRP genotype in PTSD-related phenotypes, the number of subjects for whom we had data on CRP level was limited, so we lacked adequate statistical power to examine mediation between CRP genotype, CRP level, fear physiology, and PTSD symptoms.
Our findings describe a SNP within the
CRP gene that is associated with increased susceptibility to a PTSD diagnosis and greater PTSD symptoms in our inner-city, primarily African American population. This finding joins those of a group of previous reports based on this growing cohort linking distinct candidate genes to increased risk for psychopathology and altered psychophysiology. Genetic loci within genes critical for the neuroendocrine regulation of the stress axis have been associated with increased risk for psychopathology. A SNP within the corticotropin-releasing hormone (CRH) receptor 1 (CRHR1) gene is associated with increased depressive symptoms in individuals who have experienced childhood trauma (
47). Polymorphisms within the
FKBP5 gene, an important regulator of glucocorticoid negative feedback, increase risk for PTSD symptoms in individuals exposed to childhood trauma (
48). Additionally, a polymorphism in the receptor gene (
ADCYAP1R1) for pituitary adenylate cyclase-activating polypeptide (PACAP), a peptide implicated in stress-related behavior and physiology (
49–
51), is associated with PTSD in a sex-dependent manner (
52). The
ADCYAP1R1 SNP is predictive of PTSD diagnosis and symptoms as well as dysregulated fear discrimination in females (
52). In complement to this finding, we also found that a polymorphism within the testosterone metabolic pathway, SRD5A2, was associated with PTSD only in males (
53). Results from these retrospective candidate gene studies, together with data from the present study, suggest that SNPs within genes that regulate neuroendoimmunological function confer increased risk for psychopathology, and PTSD in particular, in individuals exposed to psychosocial stressors. In the future, we aim to assess in a prospective manner whether these genetic loci confer increased risk for PTSD and to utilize genome-wide association study approaches.
This study has some limitations that should kept in mind when interpreting the results. Because the study was cross-sectional in nature, it does not allow for the determination of causality. Additionally, the study was not able to account for potential confounders of the association between CRP level and PTSD, including smoking status (
54), cardiovascular disease (
20), and obesity (
55), as these measures were not obtained in the large cohort. However, our genetic data linking the rs1130864 SNP in the
CRP gene to increased CRP levels and increased PTSD symptoms suggest that heightened CRP levels due to a genetic trait increase PTSD symptoms in trauma-exposed individuals in a manner independent of other experiential factors. The notion that baseline CRP levels influence PTSD symptoms is corroborated by recent prospective data indicating that baseline CRP level is predictive of PTSD following deployment in a military cohort (
17).
While the homogeneity of the study population is a strength of this study, it could also serve as a limitation, as the results may not be generalizable to other samples. Furthermore, our inner-city hospital sample is not a population-based sample, and thus selection biases could be present in the small subset of individuals who participated in CRP level and fear-potentiated startle analyses, which limits the generalizability of our study, as these individuals self-selected to participate further. However, genetic associations with PTSD and depression that were demonstrated in previous initial studies from this cohort (
48,
52) have been replicated in a variety of other populations of varied trauma status, race, and socioeconomic status (
56,
57). Additionally, high rates of trauma exposure (
21) and inflammation (
58) are prevalent in urban, low-socioeconomic-status, African American communities and may lead to adverse health outcomes via dysregulation of the stress-immune system, as evidenced by findings also indicating that African Americans (
58) and individuals of low socioeconomic status (
59) have elevated CRP levels. Our data suggest a possible therapeutic target for alleviating the symptoms associated with PTSD in this population, similar to what has been described in some types of depression (
60).