Anxiety has a strong familial component (
1). Children of affected parents show an increased risk for emotional problems compared with the normal population (
2). While the precise mechanism through which vulnerability is transmitted remains unclear, there is evidence that antenatal maternal emotional well-being associates with alterations in the uterine environment (
3) and in fetal physiology (
4). Antenatal maternal anxiety and the accompanying alterations in the uterine environment also predict an increased risk for childhood behavioral and emotional problems (
3), decreased gray matter density of prefrontal and sensory cortices in childhood (
5,
6), and alterations in performance on prefrontal dependent tasks (
5). Importantly, the associations of antenatal maternal anxiety with childhood emotional function (
7) and early brain growth, particularly in structures that are implicated in the regulation of emotional states (
8), are independent of postnatal maternal anxiety. These findings appear to reflect a prenatal, transgenerational transmission of individual differences in vulnerability for emotional problems.
Vulnerability for anxiety disorders in children of affected parents may also be moderated through genetic variations (
9). The catechol-
O-methyltransferase (
COMT) gene, located on chromosome 22q11.2, is well expressed across the brain before birth. The
COMT gene starts its expression in the prefrontal cortex at infancy and peaks during early adulthood (
10,
11). It regulates catecholamine signaling, particularly dopamine signaling, in the prefrontal cortex and is associated with the development of the prefrontal anatomy and function (
12). It has been suggested that
COMT variants are molecular genetic markers associated with anxiety, pain, and stress responsivity (
13). A functional single-nucleotide polymorphism (SNP) of the
COMT gene (rs4680) leads to an amino acid change from valine (
val) to methionine (
met) at position 158 (
val158
met) (
14). The
val allele is suggested as a predominant factor that determines higher COMT activity in the prefrontal cortex, which presumably leads to lower synaptic dopamine levels through enhanced degradation (
15). The
COMT genotype is associated with morphological changes of prefrontal, temporal, and superior parietal regions in neonates (
16) and adolescents (
17). The
COMT val polymorphism associates with altered processing of emotional stimuli in brain regions such as the prefrontal cortex and amygdala, which are implicated in anxiety disorders (
18). Thus, the
COMT val polymorphism is linked to an increased risk for early-onset major depression (
19), panic disorders (
20), and anxiety in adolescents and adults (
9,
21). Recently, Sheikh et al. (
22) found that even during early childhood, children homozygous for the
val allele show higher levels of internalizing symptoms compared with children with at least one copy of the
met allele. Interestingly, some studies based on Asian populations did not replicate the association of the
COMT val allele with anxiety-related disorders (
23). Moreover, Baumann et al. (
9) found that individuals homozygous for the
COMT met allele with adverse childhood experiences show higher anxiety sensitivity, which is also supported by findings from the meta-analysis conducted by Domschke et al. (
24). One reason for this discrepancy could be the result of differences in populations.
In addition to
val158
met, sequence variants lying in other loci within the
COMT gene affect gene function, ultimately resulting in altered enzyme activity and adding complexity to the functional and clinical implications of
COMT variations. Variation of the
COMT val158met (rs4680) polymorphism with the upstream SNP in intron 1 (rs737865), and the downstream SNP in the 3′ untranslated region (rs165599), has been implicated in anxiety (
25–
27). This three-SNP haplotype (rs737865-
val158
met-rs165599) differentially alters the expression of the
val158
met alleles in human brain tissue (
14). In addition, variations of this haplotype show a statistically stronger impact on prefrontal functions than the three individual SNPs, suggesting the importance of complex interactions of functional variations in
COMT in the human brain in anxiety (
28). A
COMT haplotype comprised of
val158met and rs737865 associates with high neuroticism and low extraversion, which are characteristics of anxiety-prone individuals (
26).
A prospective, longitudinal birth mother-offspring cohort study, GUSTO (Growing Up in Singapore Toward Health Outcomes), provided a unique opportunity to examine whether
COMT genotype moderates the association between antenatal maternal anxiety and in utero brain development. In this study, neonatal brain morphology was characterized by cortical thickness measured from the T
2-weighted MRI of the brain acquired shortly after birth. We predicted that individual SNPs of the
COMT gene (rs737865,
val158
met, and rs165599) would modulate the associations between antenatal maternal anxiety and cortical thickness in neonates, particularly in the prefrontal cortex. Furthermore, we predicted that haplotype analysis would be superior in predicting such effects compared with individual SNPs. We first estimated the rs737865-
val158
met-rs165599 haplotype for each participant through an expectation-maximization algorithm (
29). We then explored haplotype trend regression analysis on the neuroimaging data, where estimated haplotype probabilities were used to increase statistical power and generate findings unbiased to samples with uniquely determined haplotypes. The present study provides, to our knowledge, the first direct analysis of the genetic and environmental interactions associated with neonatal brain morphology and evidence that
COMT may represent a molecular genetic marker moderating differential sensitivity to antenatal maternal anxiety.
Method
Participants
One-hundred and eighty-nine infants of mothers who participated in the prospective GUSTO birth cohort study were recruited for neuroimaging. The GUSTO cohort consisted of pregnant Asian women attending the first-trimester antenatal ultrasound scan clinic at the National University Hospital and the KK Women’s and Children’s Hospital in Singapore. Birth outcome and pregnancy measures were obtained from hospital records. The parents were Singapore citizens or permanent residents of Chinese, Malay, or Indian ethnic background. Socioeconomic status (household income) was extracted from survey questionnaires during pregnancy. This study only includes neonates with gestational age ≥37 weeks, birth weight >2,500 g, and a 5-minute Apgar score ≥9. The GUSTO cohort study was approved by the National Healthcare Group Domain Specific Review Board and the SingHealth Centralized Institutional Review Board. Written consent was obtained from the mothers.
State-Trait Anxiety Inventory Scale
The State-Trait Anxiety Inventory (Form Y-2) was administered at 26 weeks of pregnancy to quantify the maternal prenatal level of anxiety. The State-Trait Anxiety Inventory is a commonly used self-report tool for assessing anxiety and consists of two subscales (state and trait anxiety), each containing 20 items on a 4-point rating scale. State anxiety reflects a “transitory emotional state or condition of the human organism that is characterized by subjective, consciously perceived feelings of tension and apprehension and heightened autonomic nervous system activity.” State anxiety may fluctuate over time and can vary in intensity. In contrast, trait anxiety denotes “relatively stable individual differences in anxiety proneness” and refers to a general tendency to respond with anxiety to perceived threats in the environment.” Items that assess the absence of anxiety are reverse scored. The state and trait anxiety scores can be directly computed as the sum of scores of each item and range from 20 to 80. They have a direct interpretation: high scores on their respective scales mean more trait or state anxiety, and low scores mean less. In regard to the perinatal population, the State-Trait Anxiety Inventory has been shown to have construct validity (
30). The reliability of this screening scale was 0.91 for both the state and trait subscales assessed using Cronbach’s analysis for our cohort. Several studies have suggested that maternal anxiety trait tends to remain relatively stable over the course of pregnancy (
31,
32). Hence, only the trait measure at 26 weeks of pregnancy was used in this study to examine the prenatal maternal anxiety.
MRI Acquisition and Analysis
One-hundred and eighty-nine neonates underwent axial fast spin-echo T2-weighted MRI (repetition time=3,500 ms; echo time=110 ms; field of view=256 mm×256 mm; matrix size=256×256; 50 axial slices with 2.0-mm thickness) at 5 to 17 days of life using a 1.5-Tesla GE scanner (General Electric Co., Fairfield, Conn.) at the Department of Diagnostic and Interventional Imaging of the KK Women’s and Children’s Hospital. We obtained two acquisitions of the axial T2-weighted MRI while participants were sleeping in the scanner. No sedation was used, and precautions were taken to reduce exposure to the MRI scanner noise. A neonatologist was present during each scan. A pulse oximeter was used to monitor heart rate and oxygen saturation throughout the entire scan. All brain scans were reviewed by a neuroradiologist (M.V.F). To determine image motion, all axial slices of the T2-weighted MRI data were visually inspected to ensure no cross-slice motion and checkerboard patterns.
Within individual participants, when possible, two T
2-weighted MRI acquisitions were first rigidly aligned and averaged to increase signal-to-noise ratio. In cases in which only one scan was acquired, data from one scan were used in lieu of the average axial image. The skull of the averaged axial image was removed using the Brain Extraction Tool method (
33). A Markov random field model was used to automatically delineate gray matter, white matter, and CSF from the neonatal T
2-weighted MRI data. The mathematical model of Markov random field has been described in detail by Fischl et al. (
34). The Markov random field model has been considered as one of robust automatic brain segmentation approaches because it incorporates the anatomical prior information obtained from a manually labeled training set. In our study, our training set consisted of 20 T
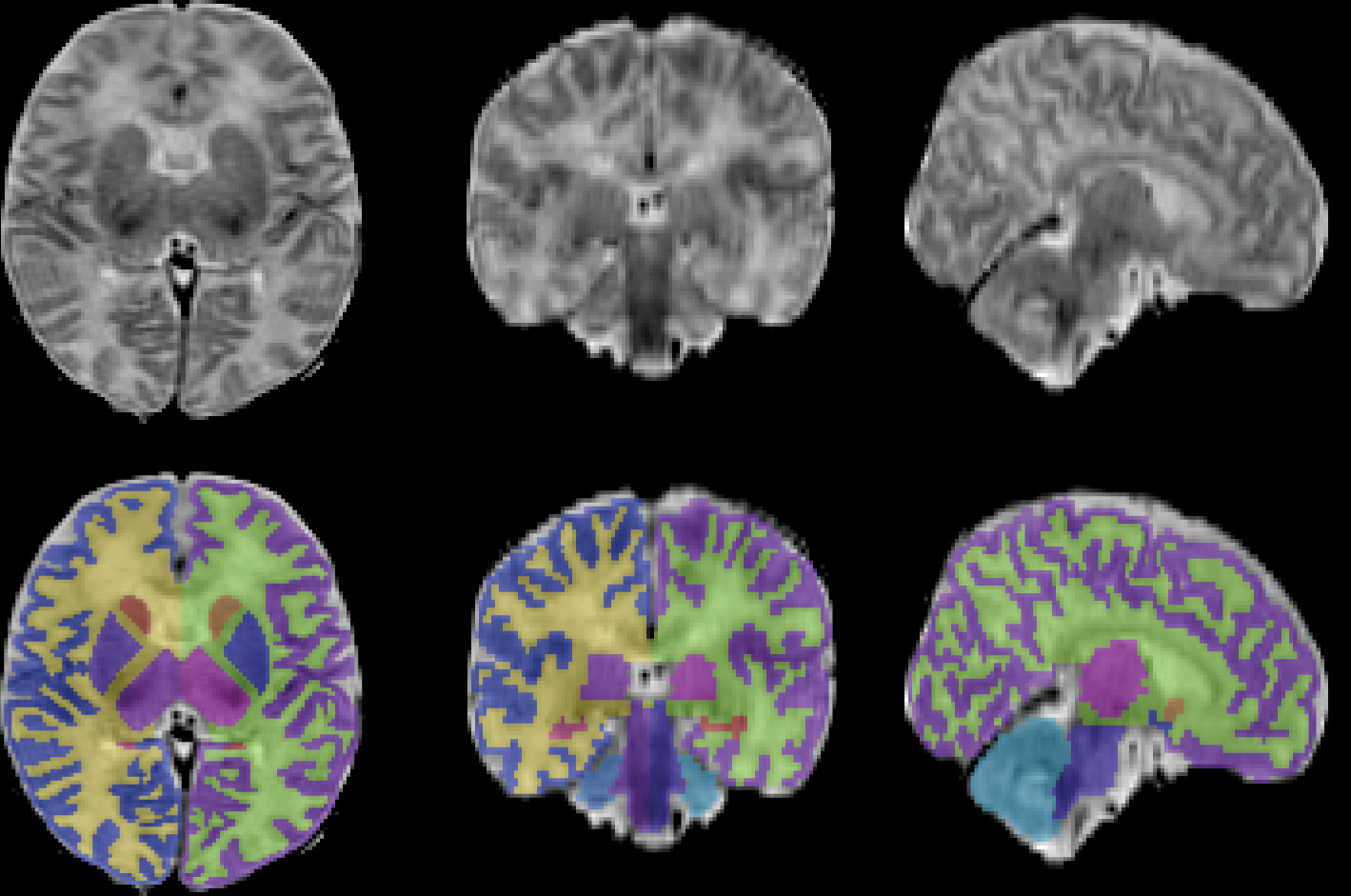
2-weighted MRI data sets randomly selected from our sample. We employed the leave-one-out validation approach to evaluate the Markov random field segmentation accuracy for gray and white matter. The 19 images with the manual label were used as training sets in Markov random field, and one image with the manual label was used as a testing set. The accuracies of the Markov random field segmentation for gray and white matter were 0.793 and 0.862, respectively. An example of the infant brain image and its segmentation is presented in
Figure 1.
Based on the above tissue segmentation, a cortical surface was constructed at the boundary between gray and white matter using a graph-based topology correction algorithm (
35). The cortical thickness was measured as the distance between the cortical surface and gray matter voxels at the boundary between gray matter and CSF. The cortical thickness was smoothed using the Laplace-Beltrami basis functions on the cortical surface. For group comparison of the cortical thickness, we employed a large deformation diffeomorphic metric mapping algorithm (
36) to align individual cortical surfaces to the atlas that was generated based on the cortical anatomy of the same 20 participants (
37,
38) and transferred the thickness of each individual participant to the atlas.
SNP Genotyping
Genomic DNA was extracted from frozen umbilical cord specimens for infants and from blood for mothers according to standard procedures. Then, the samples were genotyped on Illumina Omni express arrays (Illumina, San Diego), which were recently shown to perform well and have better coverage compared with competitors in Asian populations (
39), and on Illumina Exome arrays following the manufacturer’s instructions by Expression Analysis, Inc. (Durham, N.C.). Data were processed in the GenomeStudio Genotyping module (Illumina, San Diego). Genotyping calls were made using the GenCall software (Illumina, San Diego), which incorporates a clustering algorithm (GenTrain) and a calling algorithm (Bayesian model). The GenCall score of each SNP probe and call rate of each sample were generated. The GenCall score is primarily designed as a means by which to rank and filter out failed genotypes (
40). Scores below 0.15 generally indicate failed genotypes, and hence the genotypes with a GenCall score <0.15 are not assigned genotypes (
40).
COMT SNPs rs4680, rs737865, and rs165599 were then extracted and used in this study. Their minor allele frequencies were >0.05. The three SNPs were also not deviated from Hardy-Weinberg equilibrium (p<0.001).
Linkage Disequilibrium and Haplotype Estimation
To measure linkage disequilibrium between SNPs, we estimated the haplotype frequencies through use of the expectation-maximization algorithm on individual genotypes and calculated Lewontin’s D′ and Pearson’s correlation using Haploview software, version 4.2 (
41). We then constructed likely three-SNP haplotypes in our samples on the basis of the three polymorphisms using PHASE software, version 2.1.1 (
29). Only haplotypes with an estimated frequency >3% were used in the analysis with imaging.
Statistical Analysis
The thickness data were smoothed on the cortical surface using a 30-mm full-width-at-half-maximum Gaussian filter (
42). Linear regressions were first used to examine interactive effects of individual SNPs with antenatal anxiety on cortical thickness. The antenatal maternal State-Trait Anxiety Inventory score and the genotype of individual SNPs were included as main factors, which were entered into the first block of linear regressions, along with sex, birth weight adjusted by gestational age, and postconceptual age on the day of MRI. Monthly household income and ethnicity were separately entered into the first block to avoid multicollinearity. The interaction of the two main factors was then entered into the second block of the regression. Statistical results at each surface vertex were thresholded at the level of significance (p<0.001) and then corrected for multiple comparisons at the cluster level of significance (p<0.05). The significance of each cluster was determined using random field theory as described by Chung et al. (
43). Additional analysis was performed while controlling for the maternal genotype. Post hoc analyses were further used to examine associations of antenatal maternal anxiety and cortical thickness in each genotype group, where cortical thickness was computed as mean thickness in the brain region with the significant interactive effects.
Haplotype trend regressions were then used to test for interactive effects of haplotypes and antenatal anxiety on cortical thickness (
44). The haplotypes were represented by their estimated frequency of individual participants using PHASE software (
29). The other factors in the regression were the same as mentioned above. Statistical results were corrected for multiple comparisons at the cluster level of significance (p<0.05).
Birth weight was adjusted based on gestational age, which was determined using linear regression, with the mean-centered gestational age as a main factor in the GUSTO cohort. The residual was defined as adjusted birth weight that is statistically independent of gestational age.
Results
Demographic Characteristics
Among 189 neonates who underwent MRI scans, our study excluded neonates with gestational age <37 weeks (N=13), with birth weight <2,500 g (N=11), with a 5-minute Apgar score <9 (N=2), with large motion on the T2-weighted MRI (N=5), with no maternal anxiety measure (N=4), and with no genetic data (N=8). Thus, the total sample examined in this study was 146.
Demographic characteristics of mothers and infants are presented in
Table 1. Antenatal maternal anxiety did not vary as a function of infant sex (t=–0.39, df=144, p=0.70) or ethnicity (F=1.88, df=2, 143, p=0.16). Importantly, antenatal maternal anxiety also did not differ as a function of infant genotype: rs737685 (F=1.87, df=2, 143, p=0.16),
val158
met (F=0.66, df=2, 143, p=0.52), rs165599 (F=0.27, df=2, 143, p=0.85), showing that maternal anxiety was independent of infant
COMT genotypes. However, antenatal maternal anxiety was negatively correlated with monthly household income (Pearson's r=–0.37, df=146, p<0.001). Monthly household income was highly correlated with ethnicity (Pearson’s r=–0.23, df=146, p=0.01). To avoid multicollinearity in regression analysis, monthly household income and ethnicity were separately entered into regression analysis as covariates.
COMT and Haplotypes
All alleles were in Hardy-Weinberg equilibrium with minor allele frequency >0.05 in our sample. Distributions of the genotypes for the three SNPs among mothers and infants are presented in
Table 2. Among Chinese infants, there were 46 with AA, 15 with AG, and four with GG of rs737865; there were five
met homozygotes, 26 with
met-val, and 34
val homozygotes of
val158
met; there were 11 with AA, 34 with AG, and 19 with GG of rs165599 (data were missing for one infant). Among Malay infants, there were 33 with AA, 23 with AG, and one with GG of rs737865; there were four
met homozygotes, 22 with
met-val, and 31
val homozygotes of
val158
met; there were 15 with AA, 23 with AG, and 19 with GG of rs165599 (data were missing for one infant). Among Indian infants, there were 12 with AA and 12 with AG of rs737865; there were five
met homozygotes, 12 with
met-val, and seven
val homozygotes of
val158
met; there were seven with AA, 11 with AG, and six with GG of rs165599. Although meta-analysis suggested differences in the
COMT genotype distribution among different ethnic groups (
24), our study did not reveal ethnic group differences in the distributions of rs737865 (χ
2=8.93, df=4, p=0.06),
val158
met (χ
2=6.76, df=4, p=0.15), and rs165599 (χ
2=4.34, df=4, p=0.63).
In infants, Lewontin’s disequilibrium coefficients, D′, were 0.32 between rs737865 and
val158
met, 0.43 between rs737865 and rs165599, and 0.77 between
val158
met and rs165599, respectively. These three SNPs form seven common haplotypes with frequencies >0.03 (
Table 2).
Effects of Antenatal Maternal Anxiety
Our analyses revealed no main effects of antenatal maternal anxiety on neonatal cortical thickness after adjusting for sex, birth weight adjusted by gestational age, postconceptual age on the day of MRI, and monthly household income. However, the regression analysis adjusted for sex, birth weight adjusted by gestational age, postconceptual age on the MRI day, and monthly household income revealed that the association between antenatal maternal anxiety and neonatal cortical thickness was modulated by the
COMT genotypes of
val158
met, rs737865, and rs165599 in different anatomical regions. The interaction effect between antenatal maternal anxiety and
val158
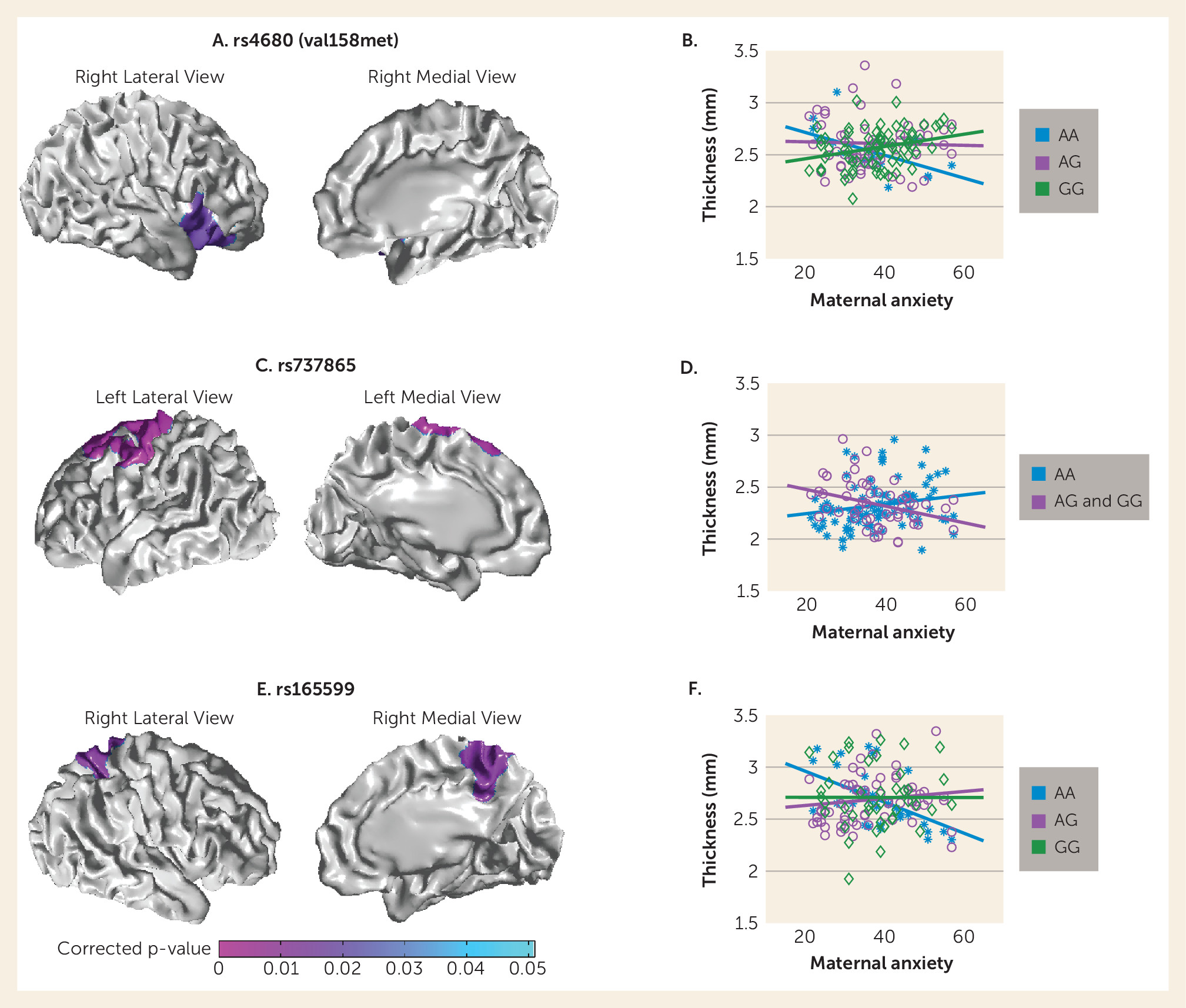
met SNP was observed in the right ventrolateral prefrontal cortex (Brodmann’s areas [BAs]: 44, 45, 47;
Figure 2A). The cortical thickness in these regions was negatively associated with antenatal maternal anxiety in
met homozygotes (t=–2.74, df=8, p=0.03) but positively associated with antenatal maternal anxiety in
val homozygotes (t=3.33, df=66, p=0.001,
Figure 2B). For rs737865, the interactive effect was observed in the left precentral gyrus and dorsolateral prefrontal cortex (BAs: 4, 6, 8;
Figure 2C). The cortical thickness in these regions was negatively associated with antenatal maternal anxiety in G carriers (t=–3.61, df=44, p=0.001;
Figure 2D). Furthermore, the interactive effect between antenatal maternal anxiety and rs165599 was observed in the right superior parietal cortex, precuneus, and posterior cingulate (BAs: 7 and 23;
Figure 2E). The cortical thickness in these regions was negatively associated with antenatal maternal anxiety in A-allele homozygotes (t=–4.11, df=27, p<0.001) but was positively associated with antenatal maternal anxiety in the AG group of rs165599 (t=2.57, df=62, p=0.01;
Figure 2F).
Our findings remained the same when controlling for ethnicity instead of monthly household income. Moreover, our findings presented in
Figure 2 were not influenced by maternal genotype.
Modulation Effects of COMT Haplotypes
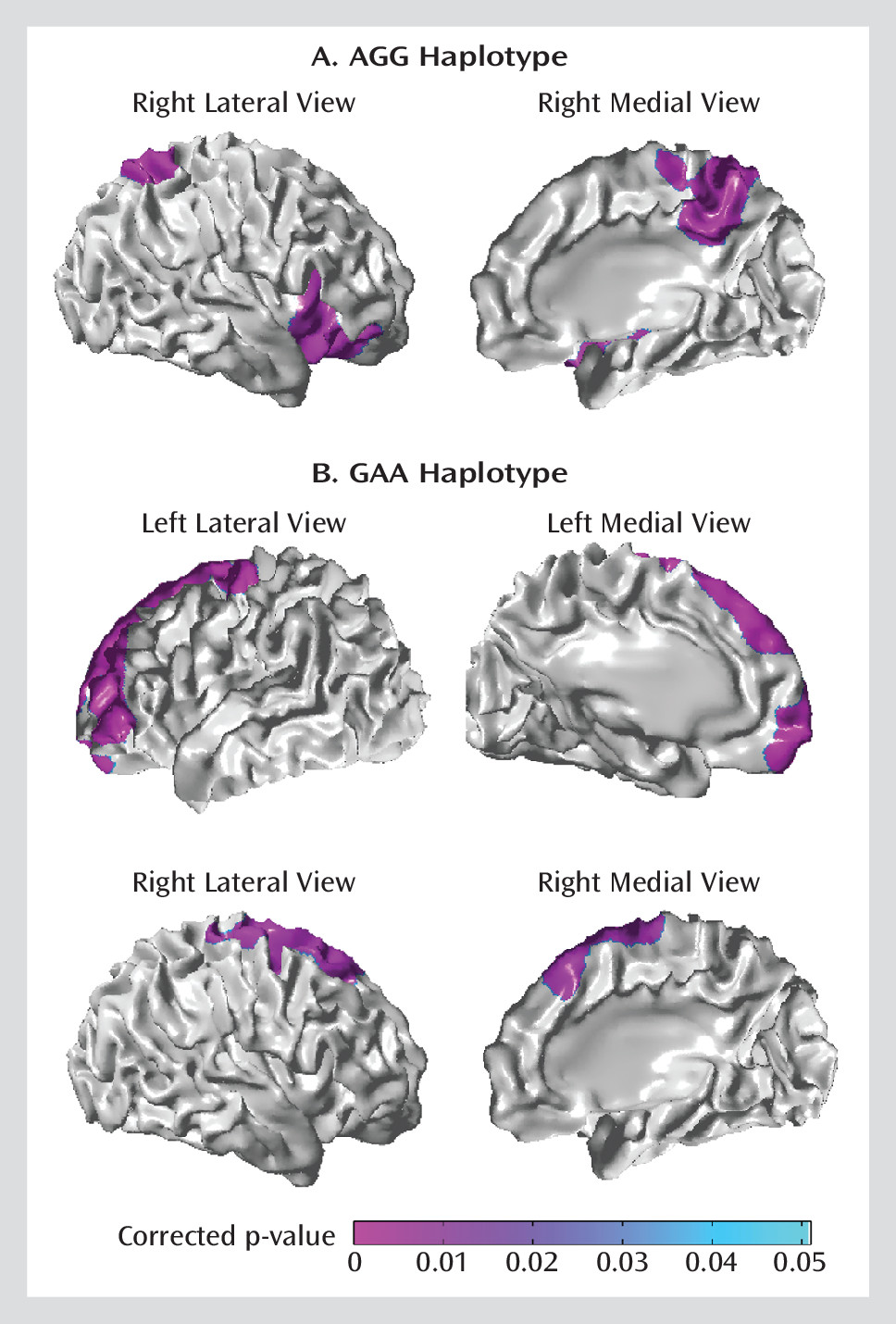
The haplotype trend regression analysis, adjusted for sex, birth weight adjusted by gestational age, postconceptual age on the day of MRI, and monthly household income (or ethnicity) revealed that associations between antenatal maternal anxiety and cortical thickness of neonates were modulated by the rs737865-
val158
met-rs165599 haplotypes. The A-
val-G (AGG) haplotype probabilities modulated positive associations of antenatal maternal anxiety with cortical thickness in the right ventrolateral prefrontal cortex (BAs: 44, 45, 47) and right superior parietal cortex and precuneus (BAs: 7 and 23;
Figure 3A). In contrast, the G-
met-A (GAA) haplotype probabilities modulated negative associations of antenatal maternal anxiety with cortical thickness in the bilateral precentral gyrus and dorsolateral prefrontal cortex (BAs: 4, 6, 8, 9;
Figure 3B).
Discussion
This study revealed that functional variants in COMT regulate associations between antenatal maternal anxiety and neonatal cortical morphology, particularly in the frontal and parietal regions. This effect can be characterized by allelic variation in individual SNPs (val158met, rs737865, and rs165599) and their haplotypes. Our results suggest that the association between maternal anxiety and in utero neurodevelopment is modified through complex genetic variation in COMT. Such genetic moderation may explain, in part, the variation in offspring phenotypic outcomes associated with maternal anxiety traits.
Our study first highlighted an association between antenatal maternal anxiety and right ventrolateral prefrontal cortex structure that is moderated by
val158
met COMT. The
met homozygous infants born to mothers with higher levels of antenatal anxiety showed cortical thinning in the right ventrolateral prefrontal region, while the
val homozygous infants born to mothers with similarly high levels of antenatal anxiety showed cortical thickening. These findings reveal a dramatic genotypic moderation of a common in utero environmental condition. Previous studies showed reduced gray matter volume in the lateral prefrontal cortex as a function of increased antenatal maternal anxiety (
6). Heightened antenatal anxiety predicts poor cognitive control when the offspring is school age (
45), as well as difficulties in executive function in adolescence (
46). Moreover, rats born to mothers exposed to stress during pregnancy have significantly reduced dendritic length in pyramidal neurons of the ventrolateral prefrontal cortex (
47). Our findings are also consistent with those of previous studies showing that
val homozygosity is associated with improved emotional regulation of the prefrontal cortex (
12), decreased risk for anxiety disorders (
23), and reduced anxiety-related traits (
23).
Val carriers show greater gray matter volume in the prefrontal cortex compared with
met homozygotes in adulthood (
48). Imaging studies also reveal an association between the low-activity
met allele and limbic and prefrontal activation in response to aversive stimuli in healthy comparison subjects (
18). Heightened emotional reactivity in
met carriers may contribute to a higher risk for psychopathology (
49) and thus contributes to the transgenerational transmission of vulnerability for anxiety. The
met allele is less active than the
val allele, resulting in a higher level of dopamine in the prefrontal cortex in
val carriers (
50). Antenatal maternal anxiety also predicts increased basal cortisol levels in the offspring, and glucocorticoids enhance dopamine signaling in the prefrontal cortex with accompanying disruptions to executive function (
51). Rodent studies show that sustained stress-induced effects on behavior are mediated by increased dopamine signaling (
52). Moreover, emotional stimuli (i.e., angry faces) activate the right ventrolateral prefrontal cortex (
53) and, interestingly, among patients with social anxiety disorders, increased activation associates with less severe symptoms (
54). Finally, activity in the right ventrolateral prefrontal cortex associates with treatment outcome among patients with social anxiety disorders (
55), which might refer to the role of this structure in appraisal and emotional regulation in response to potential threats (
56).
Interestingly, our study also revealed the role for additional
COMT SNPs, notably rs737865 and rs165599, in moderating the relationship of antenatal maternal anxiety with dorsolateral prefrontal and parietal cortical thickness in neonates, independent of the
val158
met SNP. It is unclear why the different SNPs of the
COMT gene show such apparent variable moderation effects on the associations of antenatal maternal anxiety with distinct brain regions. Nevertheless, previous studies report that variation in prefrontal function (
28) is associated with risk for anxiety disorders (
25,
27) and with rs737865 and rs165599 when examined together with the
val158
met SNP. However, other studies show that the G-allele of rs165599 is associated with prefrontal aspects of verbal memory (
57), prefrontal executive function (
58), and working memory (
59). These findings are analogous to our findings with rs165599. G-carriers born to mothers with increased antenatal anxiety had greater thickness in the right superior parietal cortex and precuneus. The two anatomical structures receive considerable visual sensory input and are involved in episodic memory, visuospatial processing, and reflection of self and are suggested as central and well-connected hubs between parietal and prefrontal regions (
60). In addition, activation of the dorsolateral prefrontal cortex, where the association with antenatal maternal anxiety was moderated by rs737865 genotype, associates with emotional regulation (
61).
The
COMT haplotype analysis improved statistical power for the moderating effect of
COMT variants on the associations between antenatal maternal anxiety and neonatal brain morphology when compared with the single SNP analysis. The results drawn from the haplotype analysis are the combination of the findings from the analysis of individual SNPs with greater extension of the findings in the bilateral dorsolateral prefrontal cortex, a region in which gray matter volume was previously shown to be associated with antenatal maternal anxiety (
6). Moreover, dopamine acts at the dorsolateral prefrontal cortex to regulate cognitive processing (
62). Infants bearing variants associated with greater enzymatic activity (
COMT 158
val combined with the A-allele of rs737865 and G-allele of rs165599) and born to mothers with higher levels of antenatal anxiety showed thicker cortex in the right ventrolateral region and superior parietal lobule. In contrast, infants bearing low enzymatic activity variants (
COMT 158
met combined with the G-allele of rs737865 and A-allele of rs165599) and born to mothers with completely comparable high levels of antenatal anxiety had thinner cortex in the bilateral dorsolateral prefrontal regions. Dorsolateral prefrontal connectivity is associated with individual differences in behavioral inhibition (
63). These findings suggest that in utero influences of antenatal maternal anxiety on the prefrontal cortex may contribute to the well-established relationship between antenatal maternal anxiety and childhood emotional function (
5). These findings are thus consistent with those of a previous study showing that genetic variants linked to the risk for psychiatric illness in later life are associated with cortical structure in infants (
16) and underscore the importance of the perinatal period of neurodevelopment for the study of individual differences in the risk for psychopathology (
64).
The strengths of this study include a unique opportunity to examine interactive effects of genes and antenatal maternal anxiety on neural development in neonates without postnatal influences, such as postnatal parenting (
65–
68). Second, the study of haplotypes, which characterize the linkage disequilibrium structure of several markers, is considered highly advantageous, since haplotypes may show stronger association with phenotype than individual markers because they contain more information about the underlying functional genetic variants. Using estimated haplotype probability in statistical analysis on imaging data increases statistical power and generates results unbiased to samples with uniquely determined haplotypes. A weakness of the present study is that antenatal maternal anxiety was only assessed at one time point during pregnancy. However, the mid-trimester is a critical period when neural migration and synaptogenesis of the fetal brain rapidly develop. Maternal trait anxiety tends to remain stable over the course of pregnancy (
31,
32,
69). Moreover, our assessment of maternal anxiety was based on a common screening tool intended to elicit a subjective report of emotional well-being but does not constitute clinical assessment. The variations in brain structure of the offspring are thus best considered as being associated with self-reported anxious symptoms and not with clinical depression, per se. Furthermore, even though previous studies have suggested that
COMT influence on brain functions varies by sex, our study did not reveal any interactive effects of
COMT and sex or main effects of sex on the thickness in the brain regions presented in
Figures 2 and
3 (all p values >0.1). Nevertheless, sex was entered as a covariate in all the analyses in this study. Finally, perhaps the major limitation of our study, and one common to most studies of imaging genetics, is our current lack of knowledge of the relationship between the genetic variants and region-specific variation in
COMT gene expression and dopamine signaling. Nevertheless, our findings are consistent with the apparent increased level of dopamine signaling in the perinatal primate prefrontal cortex (
70,
71).
In conclusion, these findings are the first, to our knowledge, to reveal that antenatal anxiety interacts with functional genotypes of COMT, resulting in alterations in the prefrontal cortical morphology of offspring. Our results suggest that an increased risk for anxiety may be transmitted from mother to child during fetal life and that the effect is dependent upon infant COMT genotype.
Acknowledgments
The authors thank the GUSTO (Growing Up in Singapore Toward Health Outcomes) study group and the clinical and home visit staff, as well as the participants of this study. Members of the GUSTO study group include: Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Mary Foong-Fong Chong, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Keith M. Godfrey, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D. Holbrook, Chin-Ying Hsu, Hazel Inskip, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Yung Seng Lee, Ngee Lek, Sok Bee Lim, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Cheryl Ngo, Krishnamoorthy Naiduvaje, Wei Wei Pang, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu-E Soh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Mya Thway Tint, Hugo P.S. van Bever, Rob M. van Dam, Inez Bik Yun Wong, P.C. Wong, Fabian Yap, and George Seow Heong Yeo.