Obsessive-compulsive disorder (OCD) is a complex and heterogeneous disorder characterized by intrusive obsessions and repetitive compulsions. Its lifetime prevalence is estimated to be 1%−3% worldwide (
1). OCD leads to an enormous reduction in life quality and often follows a chronic course when not treated. There is increasing evidence from twin and family studies for small to moderate genetic effects (
2). However, reliable evidence of specific genetic alterations has not yet emerged. Overall, data point to the involvement of gene variants in the serotonergic, dopaminergic, and glutamate systems (
2). Combined, evidence suggests that OCD is genetically complex, with multiple genetic and environmental factors contributing to its development (
2).
Hyperactive error signals that have been linked to OCD symptoms, such as feelings of incompleteness, doubt, and repetitive behavior, are assumed to play a central role in the pathophysiology of OCD (
3). The anterior cingulate cortex is implicated in the processing of conflict and the generation of error signals (
4,
5). Converging evidence from neuroimaging studies conducted at rest as well as during symptom provocation suggests that the anterior cingulate cortex as part of a frontal-striatal circuit is involved in the pathophysiology of OCD (
2,
6). Furthermore, functional MRI (fMRI) studies point toward enhanced error- and conflict-related activity in the anterior cingulate cortex in OCD patients (
7,
8). Consistent with this, the error-related negativity, a fronto-central event-related brain potential generated by the anterior cingulate cortex following an error (
9), has been repeatedly found to be enhanced in OCD patients (
10) across all symptom dimensions and unrelated to symptom severity (
11). The magnitude of the error-related negativity has also been shown to be heritable (
12), and larger amplitudes are evident in unaffected relatives of OCD patients (
13,
14). Consequently, overactive error monitoring has been proposed as an endophenotype for OCD (
14,
15). Endophenotypes are quantitative biological or cognitive markers that more closely relate to the genetic underpinnings than the clinical syndrome (
16). To qualify as an endophenotype, a marker must 1) be associated with the illness, 2) be heritable, 3) be found in unaffected family members at a higher rate than in the general population, and 4) be state independent (
16). Although enhanced performance monitoring in OCD fulfills the majority of these criteria, data evaluating its stability over time is still missing for adult patient populations. Evidence from pediatric OCD patients suggests that enhanced error-related brain activity persists after symptom reduction (
17,
18). The purpose of the present study was to examine performance monitoring in adult OCD patients before and after symptom reduction. We used cognitive-behavioral therapy (CBT) with exposure and response prevention as an evidence-based treatment to achieve symptom reduction in OCD patients (
19). To show that overactive performance monitoring in adult patients is state independent and not a secondary result of symptom expression is essential to validate the notion that this marker is an endophenotype for OCD.
Discussion
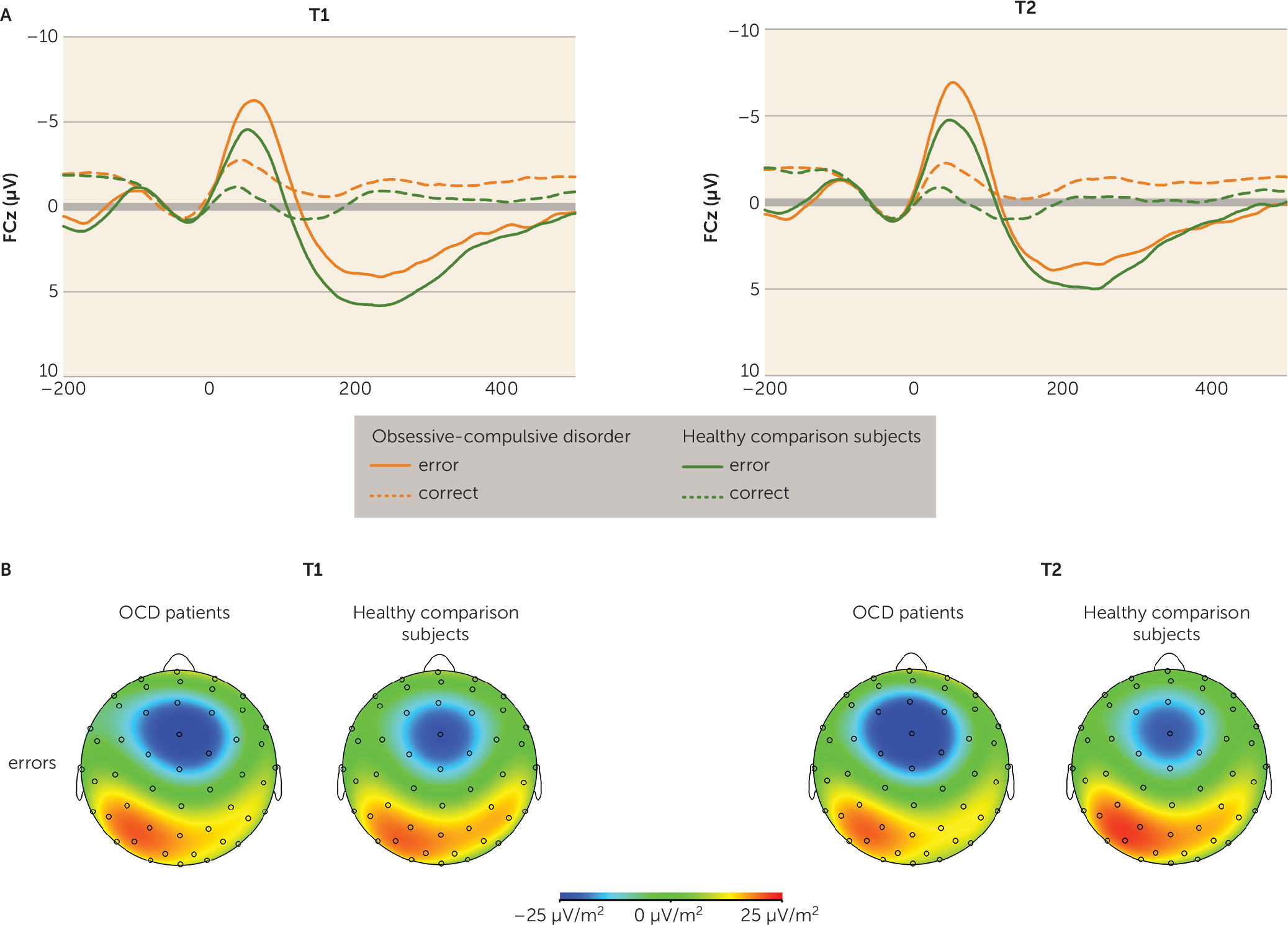
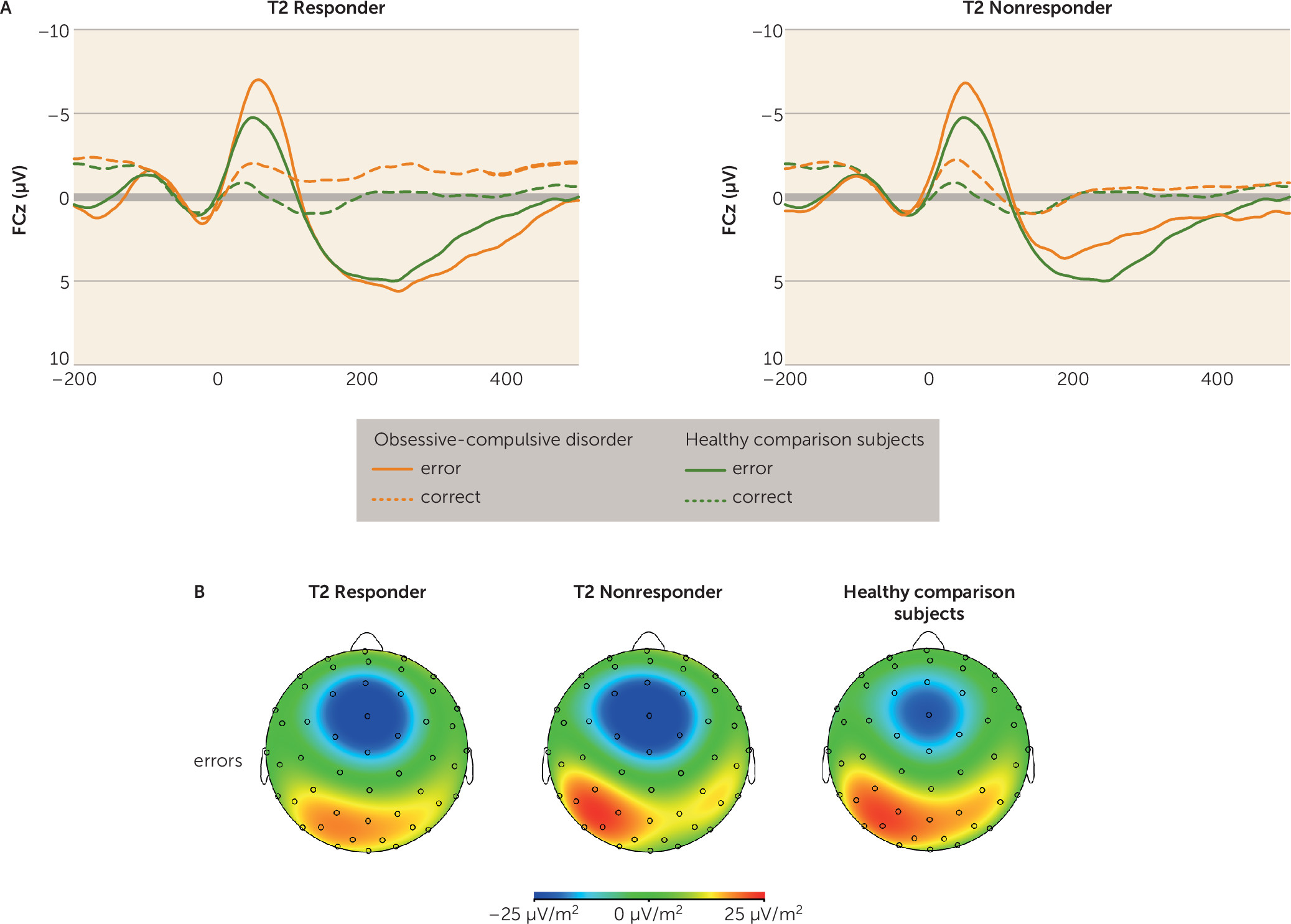
OCD patients suffer from repetitive and stereotyped behaviors that are thought to be prompted by hyperactive error signals in the brain. Overactive performance monitoring in OCD is a well-replicated finding (
10) and has been suggested as a promising endophenotype for the disorder (
13–
15). The present study aimed to further clarify the suitability of overactive performance monitoring as an endophenotype by investigating its state independency (
16). Electrophysiological indicators of performance monitoring were measured before patients received CBT and after completing it. Symptoms commonly remit to variable degrees following therapy, but an endophenotype marker should be unaffected by these symptom changes over time. Consistent with findings from previous studies, OCD patients showed enhanced neural correlates of performance monitoring prior to CBT (
10). Importantly, this alteration persisted after symptom reduction. Accordingly, both treatment responders and nonresponders continued to show enhanced neural responses to errors and correct reactions compared with healthy comparison subjects. Moreover, no correlations between symptom changes and variations in response-related brain potentials were observed. For both OCD patients and healthy comparison subjects, the error-related negativity amplitude increased between sessions, which may suggest that the significance of errors increased (
30). Alternatively, the signal-to-noise ratio may have been superior at the second session. Overall, the retest-reliability for the error-related negativity (r=0.65, p<0.001) was similar in size to previously reported values (
31), indicating that the error-related negativity is a stable, trait-like component that can be reliably measured.
The present results are consistent with results in pediatric OCD patients revealing that increased error-related negativity (
17) and increased error-related activity in the anterior cingulate cortex (
18) are maintained over the course of successful therapy. Together, these results indicate that brain correlates of overactive performance monitoring in OCD are independent of symptom state and seem to represent an underlying risk marker or endophenotype. The persistence of enhanced performance monitoring after symptom reduction is remarkable given some findings suggesting that hyperactivity of fronto-striatal brain circuits in OCD patients during resting state and after symptom provocation normalizes, at least in part, with symptom reduction during psychological or pharmacological treatment (
2,
32). Brain structures showing such functional normalization include the nucleus caudatus (
33), the thalamus (
28), and the anterior cingulate cortex (e.g., references
34,
35). Although state-dependent variations in anterior cingulate functions have been shown, overactive performance monitoring, which has been linked to activity in this region, appears to be independent of current symptom state (
17,
18). It is not entirely clear how the trait-like error-related negativity and state-like anterior cingulate cortex activity at rest and during symptom provocation are related to each other in healthy individuals and in OCD patients. Future studies should evaluate this by concurrent measures of EEG and fMRI during rest, symptom provocation, and performance monitoring in a follow-up design.
It should be noted that enhanced error monitoring has not only been found in OCD but also in depression, social anxiety, and generalized anxiety disorder, as well as in nonclinical individuals showing associated traits (
10,
36,
37). These conditions characterized by overactive error monitoring are frequently comorbid (
1). These disorders overlap in symptoms, comorbidities, and neural correlates, which suggests partially overlapping etiological factors. Consequently, overactive performance monitoring may represent an endophenotype beyond the diagnostic borders of OCD and therefore represents an interesting marker for the Research Domain Criteria (
38). This initiative tries to establish biologically meaningful dimensions of psychological dysfunction irrespective of disorder categories. Performance monitoring is a fundamental behavioral function, and its abnormalities at both ends of the distribution (i.e., enhancement and reduction) have been implicated in multiple forms of psychopathology (
15,
36). More specifically, overactive performance monitoring may be a transdiagnostic dimensional trait that is shared by individuals who are highly sensitive to the commission of errors (
10,
36).
However, enhanced performance monitoring after correct reactions may represent a more specific marker of OCD (e.g., references
11,
14,
39) that has not been reported for generalized anxiety disorder (e.g. reference
40) or depression (e.g., reference
41). The present study provides further evidence for hyperactive performance monitoring associated with both correct and erroneous responses in OCD (e.g., references
11,
14,
39). This nonspecific enhancement of performance monitoring (to both correct responses and errors) in OCD may relate to the feeling that something is wrong and needs to be repeated regardless of actual outcome and may therefore relate to repetitive behavior and compulsions. Performance-monitoring signals are assumed to trigger the adjustment of cognitive control (
42). Therefore, a permanently overactive performance-monitoring system might account for the greater urge to control actions and thoughts, a characteristic commonly observed in OCD and assumed to enhance vulnerability according to cognitive models of OCD (
43).
The present work adds important evidence for the role of performance monitoring as an endophenotype for OCD, but it has limitations that should be noted. Since a naturalistic OCD patient sample was examined, some patients were medicated and some had current comorbid disorders. However, post hoc tests showed that results were not significantly affected by medication or comorbidity in the patient group. We could not analyze the subgroup of remitted patients (Yale-Brown Obsessive Compulsive Scale score <7), since this subgroup was too small (N=7) to attain sufficient statistical power. Numerically, there was no decrease in performance monitoring in this subgroup. Future research should also explore the stability of overactive performance monitoring over longer periods of clinical stabilization.
Furthermore, it remains to be clarified whether endophenotypes mediate between genes and the clinical phenotype or are risk indicators that share genes with the clinical phenotype (
44). In addition to genes, environmental risk factors, such as aversive learning experiences regarding errors (
45), can influence both the endophenotype and the clinical phenotype. In general, evidence identifying specific genes or mechanisms by which endophenotypes drive psychopathology has been limited (
46). However, one major gain from endophenotype research may be the development of transdiagnostic concepts that are not limited to categorical diagnoses (
46) as proposed in the Research Domain Criteria (
38). Furthermore, the identification of endophenotypes enhances our understanding of the complex psychiatric disorders and can enable the early detection of individuals at risk and inspire research on new treatment strategies and targets. To examine whether performance-monitoring processes can be altered by specific interventions or training models that directly target it, and whether such changes in performance monitoring may lead to changes in symptoms as expected by a mediator model of endophenotypes (
44), is a highly interesting and important avenue for future research. The current finding that overactive performance monitoring is independent of symptom changes in adult patients with OCD further validates its role as an endophenotype indicating vulnerability for OCD.