Anxiety disorders are the most common group of disorders, with a lifetime prevalence of about 30% (
1). They are associated with a wide array of personal, financial, and societal costs (
2), making research into their etiology a priority. A notable feature of anxiety disorders is that they start early in life, with a mean age at onset of 11 years (
1). It is therefore crucial to understand the development of anxiety symptoms in young people.
One of the most robust features of anxiety disorders is that they run in families (
3). This association has been widely explored, and there is evidence for a number of contributing mechanisms. First, children and adolescents can develop symptoms of anxiety by vicarious learning, whereby offspring watching anxious behaviors in their parents then model these behaviors themselves (
4). Second, anxious mothers may have a more anxious parenting style, including holding more negative expectations and showing greater intrusiveness, overprotection, and expressed anxiety (
5,
6), although it should be noted that most studies that have found this association focused on families in which the child also had an anxiety disorder or heightened anxiety symptoms. Third, as well as parents influencing their offspring, there is evidence for the reverse effect. Specifically, studies have found that parents of offspring with anxiety disorders show higher levels of parental overprotection and rejection and lower levels of emotional warmth (
7,
8). However, these studies do not take into account another possibility, whereby genes that affect parent anxiety and child/adolescent anxiety also influence parenting, thus leading to a confounding of genetic and environmental influences.
Twin studies consistently reveal mild to moderate genetic influences on a wide range of child and adolescent anxiety measures, including trait anxiety (
9), disorder-related symptoms assessed by self-report (
10) and parent-report (
11), and anxiety disorders (
12,
13). Similarly, studies of adults reveal mild to moderate genetic influences on trait anxiety (
14), anxiety symptoms (
15), and anxiety disorders (
16). This suggests that the transmission of anxiety phenotypes from parents to their children could be explained, at least partially, by genetic influences. It is noteworthy that there is considerable genetic overlap across symptoms reflecting different anxiety diagnoses (such as separation anxiety, social anxiety, and general anxiety) in child (
17,
18), adolescent (
10), and adult (
10,
19) samples. Furthermore, epidemiological studies have found evidence for substantial heterotypic continuity within the anxiety disorders (i.e., continuity of anxiety over time does not sit within diagnostic boundaries [
20]). Thus, a child who presents with separation anxiety may develop generalized anxiety in adolescence and panic disorder in adulthood. This heterotypic continuity, combined with the genetic overlap found between different types of anxiety measures, suggests that for examination of transmission of anxiety one should use a broad measure of anxiety symptomatology reflecting general vulnerability to anxiety disorders.
In addition to providing evidence for genetic influences, twin studies also provide support for a significant role of the shared environment (
21), nongenetic influences that contribute to family members resembling one another, and the nonshared environment, that which leads to differences among family members. The contribution of shared environment to variation in anxiety is consistent with the evidence described above for the importance of family environment in the development of anxiety in children. For example, siblings may show similar levels of anxiety because they were both exposed to the same level of parental-expressed anxiety. In summary, there is evidence to support a role for both genetic and environmental influences on anxiety in adults and in children and adolescents; however, to our knowledge no study to date has explicitly tested the relative contribution of genetic and environmental influences to the transmission of anxiety within families. This is important because we might target interventions for pediatric anxiety differently if intergenerational transmission is largely a result of genes versus resulting from the family-wide environment.
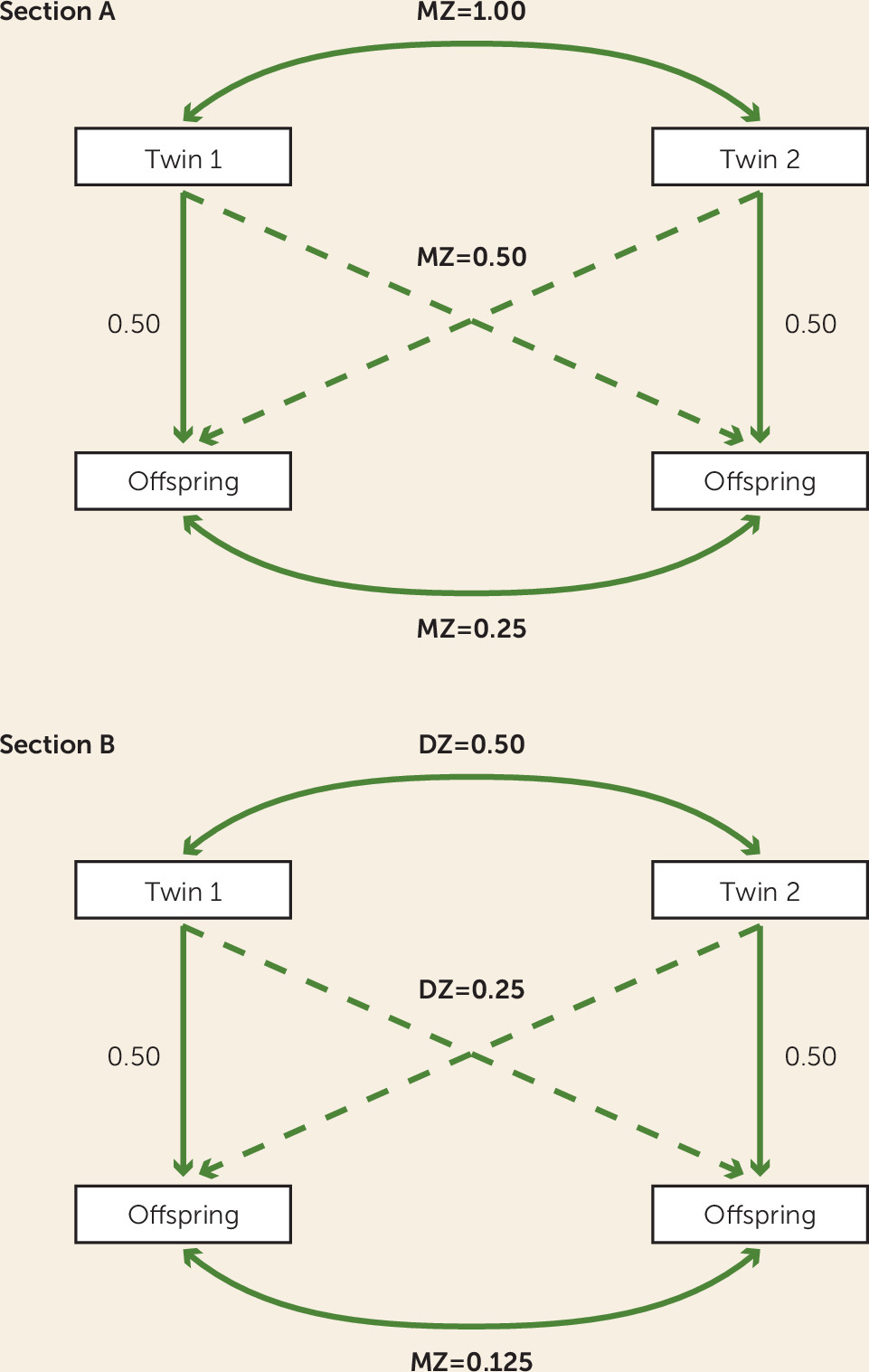
Although pediatric twin studies are able to ascertain the proportion of variance in child/adolescent phenotypes due to genetic and environmental influences, they cannot specify the extent to which genes and the environment contribute to transmission from parents to their children. For this, other genetically sensitive designs are needed. The children-of-twins model is one such design and involves adult twin pairs and their offspring (
22). By comparing correlations between children and their parent and contrasting this with correlations between children and their parent’s identical cotwin, we can learn about the influence of living with one’s parent over and above simply receiving 50% of their genes. Furthermore, by comparing the extent to which correlations between children and their twin uncle/aunt (avuncular correlations) differ for monozygotic and dizygotic twin families, we can infer the extent to which genetic and environmental factors influence transmission from one generation to another. Children share a greater level of genetic influence with their uncle/aunt when in monozygotic families than when in dizygotic families (see
Figure 1). Thus, if children resemble their uncle/aunt to a greater extent in monozygotic families than in dizygotic families, this implies a genetic influence on transmission of the trait of interest. In contrast, if these two sets of correlations are similar, and are significantly lower than the parent-child correlations, this is indicative of an environmental mode of transmission. While transmission of both depression and internalizing symptoms within families has been found to be a result of environmental transmission independent of genetic effects (
23–
25), the transmission of anxiety-related traits within families has yet to be explored using the children-of-twins design.
In the present study, we used the Children of Twins design to examine the relative contribution of genetic and environmental influences to transmission of broadly assessed anxiety from one generation to another. For this analysis, we used a measure of anxious personality in parents and a measure of anxiety symptoms in the offspring. For simplicity, from herein we use the word “anxiety” alone when referring to the transmission of parental anxious personality to offspring anxiety symptoms while specifying all other types of anxiety as applicable. As a comparison, we also repeated analyses for neuroticism, given that this personality trait shows strong phenotypic and genetic correlations with anxiety (
26).
Method
Participants
Data were drawn from the Twin and Offspring Study of Sweden and comprised information on 387 monozygotic twin families and 489 dizygotic twin families. A twin family comprised a twin pair, each twin’s spouse, and one of each of their adolescent children. Only same-sex twin pairs were used, and twin offspring were selected so that cousins were the same sex as one another and did not differ in age by more than 4 years. Thirty-seven percent of twin pairs were male, and 52% of offspring were male. At the time of data collection, the mean age of offspring in the Twin and Offspring Study of Sweden sample was 15.7 years (SD=2.4; range 11–22). The mean age of twins in the sample was 44.8 years (SD=4.9; range 32–60), and for spouses the mean age was 45.5 years (SD=5.4; range=25–65). After complete description of the study was given, written informed consent was obtained. Further information on the Twin and Offspring Study of Sweden sample is provided elsewhere (
27).
Measures
Parental anxious personality was self-reported by twins using 20 items from the Karolinska Scales of Personality (
28,
29). Items were oriented toward either social (“I often feel uncertain when I meet people I don’t know very well”) or physical (“sometimes my heart beats hard or irregularly for no particular reason”) aspects of anxiety, in addition to general worry (“I often worry about little things which others see as unimportant”). Respondents used a Likert scale ranging from 0 (not at all) to 3 (very) true. Item scores were summed to give an overall anxiety rating. Cronbach’s alpha was 0.90.
Offspring anxiety symptoms were measured using items from the Child Behavior Checklist (
30). Twins, their spouses, and offspring all reported on offspring behavior over the previous 6 months. A previous study aimed at developing DSM-oriented scales from Child Behavior Checklist items (
31) was used to guide the selection of items included in our anxiety scale, and as with the parental anxiety symptoms, the focus was on items that reflect social (“clings to adults or too dependent”) and physical (“I’m nervous, high strung, or tense”) aspects of anxiety, as well as general worry (“I worry quite a lot”). Items were scored on a scale ranging from 0 (not) to 2 (very/often) true. Items from all three reporters (which correlated from 0.25 to 0.45) were summed to create an overall anxiety score to provide the broadest and most objective measure possible. Cronbach’s alpha was 0.73.
Neuroticism was self-reported for both twins and offspring using the neuroticism scale of the Eysenck Personality Questionnaire (
32). The scale comprised nine yes/no items (such as “are you often worried without knowing why?”) scored 1/0 for yes/no. Item scores were summed to give an overall neuroticism rating. Cronbach’s alpha was 0.72 for parents and 0.67 for offspring.
Analyses
Analyses on anxiety included 385 monozygotic twin families and 486 dizygotic twin families. However, the neuroticism measure was only included in the second cohort of the study, resulting in a reduced sample comprising 222 monozygotic families and 293 dizygotic families for the neuroticism analyses.
Genetic analyses were conducted in the structural equation modeling program OpenMx (
33). Prior to analyses, residuals were taken to control for twin sex and age as is standard practice in twin analyses (
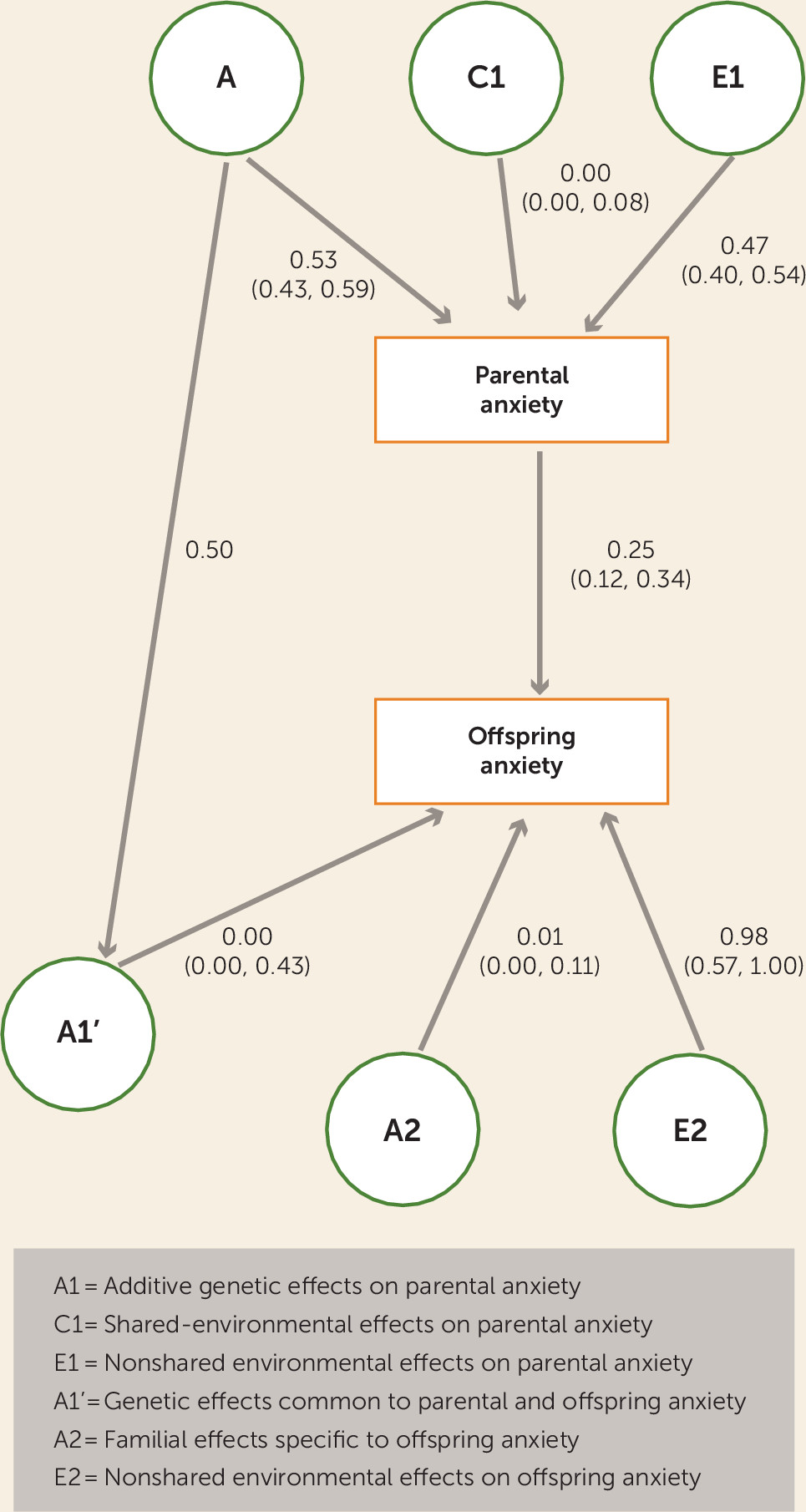
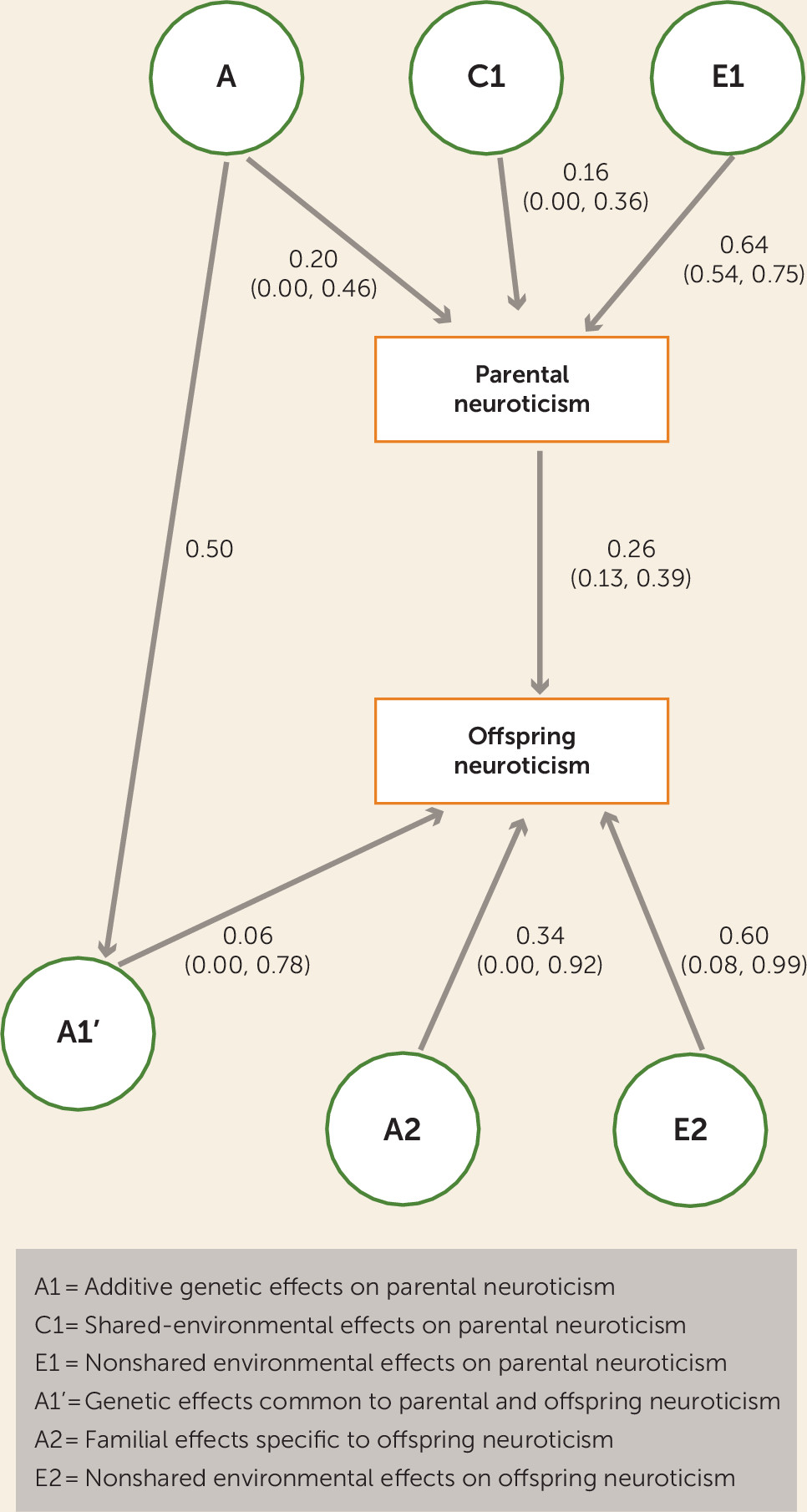
34). All variables were log-transformed to correct for skew. We fitted structural equation models to the data using maximum likelihood estimation. Models allowed us to quantify the effects of additive genetic (A), common environmental (C; nongenetic effects that make members of a nuclear family similar to one another) and nonshared environmental (E; environmental effects that make members of a family different from one another) effects on parental anxiety. By comparing the magnitude of monozygotic twin correlations (attributable to the combined effects of A+C) to dizygotic twins (attributable to 0.5*A+C), genetic and environmental influences can be estimated. Comparing monozygotic and dizygotic avuncular correlations and parent-child correlations allows for the estimation of genetic and nongenetic intergenerational pathways. Comparing correlations between cousins from monozygotic families with those of dizygotic families allows for the estimation of the role of familial and nonshared environmental effects on offspring anxiety.
The model used is shown in
Figures 2 and
3. It is noteworthy that the path from parent phenotype to offspring phenotype reflects the direct influence of a parent’s behavior on his or her offspring. Because this is manifested through the environment, we describe it as “environmental” transmission, but it should be noted that this pathway also reflects gene-environment correlation if there is genetic influence on the parent phenotype. Second, as in all children-of-twins models, there was no shared environment factor for the offspring part of the model, since this would result in there being too many paths for the data available. The significance of the environmental (from parent to offspring phenotype) and genetic (from A1′ to offspring phenotype) intergenerational pathways was tested by creating submodels in which they were fixed to zero. Both chi-square difference tests and Akaike’s information criterion were used to assess whether submodels were a significantly worse fit to the data than the full model. All analyses were repeated controlling for assortative mating, but results were substantively similar (see the online
data supplement).
Results
The correlation between the anxiety measures and neuroticism was 0.70 for parents and 0.42 for offspring. Correlations between twin parents and their offspring for both anxiety measures and neuroticism are summarized in
Table 1. Several findings from this table are worth noting. First, there is evidence for genetic effects on both anxious personality and neuroticism in the parents, as indicated by the larger monozygotic correlations compared with dizygotic correlations (e.g., 0.55 compared with 0.20, respectively, for anxiety). Second, there is evidence for genetic influence on offspring anxiety symptoms and neuroticism given the larger correlations for cousins from monozygotic families compared with dizygotic families (e.g., 0.08 compared with −0.03, respectively, for anxiety). Third, there is marginal evidence for genetic effects on transmission of anxiety given the larger avuncular correlations (offspring 1–parent 2 and vice versa) for monozygotic families compared with dizygotic families (0.11 compared with 0.02, respectively). However, these values are both small, and for neuroticism, this pattern is not seen (0.03 compared with 0.07 for monozygotic and dizygotic families, respectively). Overall, these data do not suggest substantial genetic transmission of broadly assessed anxiety or neuroticism. Finally, there is modest evidence for environmental transmission of both anxiety and neuroticism, given the larger parent-offspring than avuncular correlations in both monozygotic and dizygotic families. For example, in monozygotic families, while the avuncular correlation for anxiety was 0.11, the parent-offspring correlation was 0.20.
The fit indices from the model-fitting analyses for twin parents and their offspring for both anxiety and neuroticism are presented in
Table 2. It is noteworthy that the power to detect genetic effects in the child part of the model was low, given that the genetic resemblance between cousins from monozygotic families compared with dizygotic families was 0.25 compared with 0.125. In both cases, the model restricted to environmental transmission only, from which the genetic transmission path was removed (model 3), provides a good fit to the data indicating that genetic transmission is not a significant contributor to overall model fit. In contrast, for both anxiety and neuroticism, the genetic transmission-only model, in which the environmental transmission path was dropped, provides a significantly poorer fit to the data providing support for the environmental transmission pathway. However, given the complexity of these models, we provide the results for the full model rather than the best-fitting model (
Figures 2 and
3). Examination of the confidence intervals for the environmental transmission and genetic transmission paths for both anxiety and neuroticism revealed that the confidence interval for the environmental transmission pathway, going from parent to offspring, did not include zero. In contrast, in both cases, the genetic transmission path (from A1’ to offspring phenotype) did include zero.
Discussion
This is the first study, to our knowledge, to examine the transmission of anxiety using a children-of-twin design. These analyses provide support for the direct, environmentally mediated transmission of anxiety from parents to their adolescent offspring over and above any genetic confounding of this association. It is noteworthy that the analyses do not provide support for genetic transmission being a significant influence, given the assumptions of our model that the same genetic factors influence both adolescent and adult anxiety. These two findings will be discussed in turn.
There are three potential interpretations of the environmental influence on the association between parent and offspring anxiety. The first is that parental anxiety itself results in a rearing environment that is conducive to the development of anxiety in young people. This could operate through a number of mechanisms. One is vicarious learning (
4), whereby the young person learns to be fearful of certain environmental stimuli by observing the presence of that fear in his or her parent(s). For example, if an adolescent witnesses repeated examples of parental anxiety in response to ambiguous or only mildly threatening behavior, he or she will learn that the world is an unsafe place. Such learning can also take place through hearing explanations or interpretations of ambiguous or mildly negative experiences that emphasize the threat content of that experience, reflecting anxiety-related information processing on the part of the parent (
35,
36). In both these instances, while there may be a genetic influence on the parent’s expressed anxiety (as seen in the models here), the way in which the anxiety is transmitted is through a direct environmental pathway between the parent’s behavior and the offspring. The second interpretation of this environmental pathway is that it reflects anxious parents engaging in negative parenting behaviors that promote anxiety in their offspring. However, although anxious parents have been shown to be more likely to display overcontrolling behavior (
5,
37), and to be more negative and critical of their offspring (
5), this is most commonly found in the context of the child/adolescent displaying high anxiety. This leads to the third interpretation, which is that anxiety in the offspring influences the parenting he or she receives. This is compatible with findings that parents of anxious children/adolescents display parenting behaviors that exacerbate the anxiety symptoms (
8,
37). One study of anxious children found that associations between negative parenting behaviors and child-expressed anxiety during a task were far stronger for mothers who were also anxious compared with mothers who were not anxious (
5). This suggests that there are likely to be effects in both directions. Whatever the mechanism, the present study is the first to demonstrate that the association between parent and child anxiety persists after accounting for genetic confounding.
The lack of evidence for genetic transmission of anxiety within families may at first appear surprising, given the heritability estimates found for both anxiety and neuroticism. However, it is consistent with other findings on internalizing problems, which have similarly shown evidence only for environmental transmission (
23–
25,
38). There are at least three possible explanations for this apparent lack of genetic transmission for anxiety-related phenotypes within families. The first relates to the assumptions of our model, that the same genes influence both adult and adolescent anxiety. At present, the twin literature is inconclusive on this issue. Two studies, one of mixed anxiety/depression (
39) and the other of anxious cognitions (
40), both across adolescence and into young adulthood, identified considerable continuity of genetic factors. In contrast, two studies of mixed anxiety/depression (
41) and fears (
42) from adolescence into young adulthood found only modest stability with considerable genetic innovation. Thus, it remains unclear the extent to which the same genes affect both adolescent and adult anxiety-related phenotypes. Given this, it is plausible that while parental anxiety is genetically influenced, these genes are different from those relevant to the development of adolescent anxiety. Testing this hypothesis would require children-of-twins data on which we had measures from adolescence on the adult parent twins. Currently, no such data set exists, although the number of longitudinal child twin studies in which the children are entering young adulthood is encouraging from this perspective (
43,
44). Second, it is also possible that the heritability estimate for anxiety phenotypes obtained from child/adolescent twin studies includes interactions between genetic factors and the shared environment. Such interactions in a traditional twin design would be included in the heritability estimate (
45), but in the children-of-twins design we use this would not be the case because the effects of the shared environment cannot be estimated in the offspring generation. Given that shared environment is found to be significant more often in child anxiety phenotypes than in other types of psychopathology, this is another plausible explanation. Third, it is possible that measurement error reduced the paths between the generations thus reducing our ability to detect genetic transmission. However, the study used relatively standard questionnaire measures for which there is at least adequate reliability.
This study has a number of notable strengths. It is the first children-of-twins analysis of transmission of anxiety from parents to their adolescent offspring. It uses a relatively large data set that provides good statistical power. Finally, we provide an internal replication by considering neuroticism in addition to anxiety. However, there are a number of limitations. First, while the study was fairly large, one always achieves greater power with larger samples. As shown in
Table 1, there was a modest difference between the avuncular correlations for anxiety from monozygotic families compared with dizygotic families (0.11 and 0.02, respectively), which suggests modest genetic influence, that may have proved significant with a larger sample. For neuroticism, there was no indication of genetic transmission, even in the correlational data. Second, although these findings provide support for direct transmission, the use of cross-sectional data means that they do not indicate the direction of effect. Finally, it is possible that parents with higher levels of anxiety over-reported anxiety in their offspring, resulting in a bias in parent-offspring correlations at the upper end of the distribution. However, mean levels of anxiety do not differ across our monozygotic families compared with our dizygotic families, and thus any bias associated with higher anxiety should not affect our estimates of genetic and environmental influences or the direct transmission pathway.
In summary, the association between parental and offspring anxiety remains after accounting for genetic transmission. These results are consistent with a direct, environmentally mediated effect of parent anxiety on offspring anxiety or could reflect anxious adolescents eliciting anxiety in their parent. Longitudinal analyses using an extended children-of-twins design will be useful in exploring these questions further.