Functional MRI (fMRI) studies have examined most prominently hot and cool executive functions and emotion processing. fMRI studies of hot executive functions have found underactivation in youths with disruptive disorder compared with control subjects in predominantly paralimbic regions, including the orbitofrontal cortex, ventromedial prefrontal cortex, anterior cingulate (
16,
17), dorsolateral prefrontal cortex (
18), parahippocampal gyrus, caudate, thalamus, and temporal (
18–
20) and inferior parietal cortices (
19) (see Table S1A and Figure S1 in the
data supplement that accompanies the online edition of this article). Few fMRI studies have tested cool executive functions, but such studies have shown underactivation in the dorsolateral prefrontal (
21), temporo-parietal (
16,
22,
23), dorsal anterior cingulate, and limbic regions (
16) (see Table S1B in the
online data supplement).
Given the heterogeneity of disruptive behavior disorder, some studies have attempted to disaggregate brain abnormalities associated with the disorder from those linked to the DSM-5 “limited prosocial emotions” specifier, characterized by psychopathic traits of callousness, remorselessness, lack of empathy, and shallow affect (
33), or from those linked to the commonly associated attention deficit hyperactivity disorder (ADHD) comorbidity. Severity of psychopathic traits in disruptive behavior disorder has been associated with decreased activation during pain processing and with affective and hot executive functions in the dorsal anterior cingulate, ventromedial prefrontal, and striato-limbic regions (
20,
28,
34–
40), while ADHD symptoms have been associated with increased insula (
30) and decreased frontal activation during emotion processing (
41). Direct comparisons showed that youths with noncomorbid conduct disorder, relative to youths with ADHD, had disorder-specific underactivation in the ventromedial orbitofrontal cortex during hot executive functions (
16) as well as in the limbic areas of the anterior cingulate, insula, and hippocampus during cool executive functions. Conversely, youths with ADHD had disorder-specific underactivation in the inferior prefrontal and dorsolateral prefrontal cortices (
4,
16,
22,
23).
Although the majority of studies in disruptive behavior disorder point toward underrecruitment of paralimbic regions that mediate motivation and affect control, such as the ventromedial prefrontal, anterior cingulate, striatal, and temporo-limbic areas, inconsistencies in findings likely resulted from small sample sizes, heterogeneity, and comorbidity (e.g., gender, ADHD, psychopathic traits); differences in analytical methodology (e.g., whole-brain or region-of-interest analyses); and/or cognitive domains tested.
The aim of this meta-analysis was to establish the most consistent brain function abnormalities of disruptive behavior disorder using all published whole-brain fMRI studies, which do not bias findings to a priori hypothesized regions (
42). To reduce heterogeneity, sub-meta-analyses were conducted of functional subdomains of emotion processing, of hot and cool executive functions, and of patients with psychopathic traits. Furthermore, meta-regression analyses assessed effects of gender, medication, and ADHD comorbidity. Based on whole-brain fMRI findings (
Table 1; see also Table S1 in the
online data supplement), we hypothesized that youths with disruptive behavior disorder relative to control subjects would show the most consistent underactivation in paralimbic regions of motivation and affect control, such as the medial prefrontal cortex, anterior cingulate, and temporo-striato-limbic areas. Furthermore, we hypothesized that those with psychopathic traits would show more prominent deficits in striato-limbic regions (
15,
20,
28,
34–
38), while ADHD comorbidity would be associated with inferior prefrontal dysfunction (
4).
Discussion
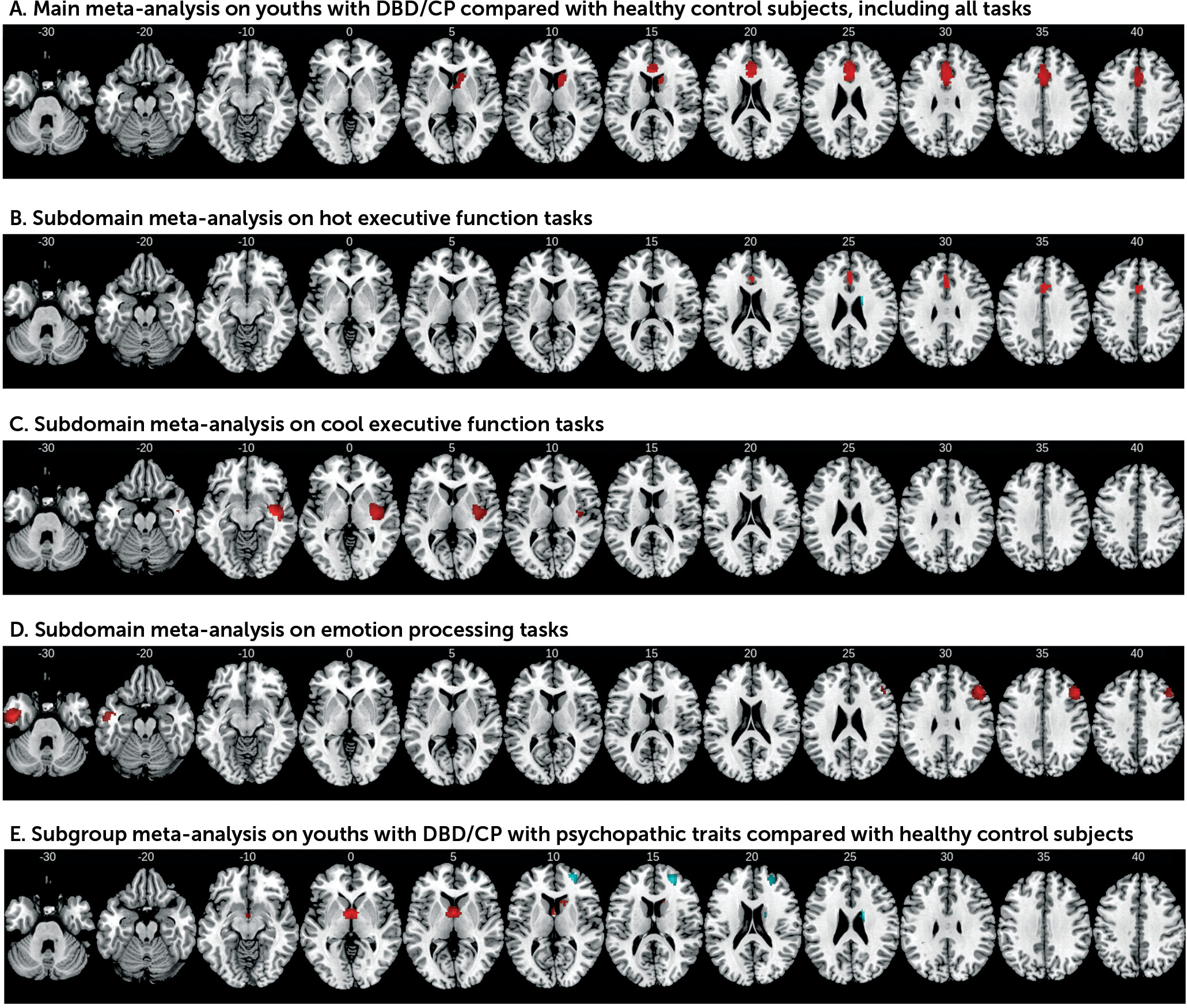
The meta-analysis across 53 whole-brain fMRI task contrasts showed that youths with disruptive behavior or conduct problems have the most consistent deficits in the closely interconnected dorsal and rostral anterior cingulate and medial prefrontal cortex involved in top-down regulation of motivation and affect, and in the ventral striatum, which is part of the same affect control network. The dysfunction in the dorsal and ventral medial prefrontal cortex largely arose from studies of hot executive function subdomains, suggesting that this dysfunction is associated with reward-related decision making.
The dorso-rostral anterior cingulate and medial prefrontal cortex, together with their close connections to the ventral striatum and limbic regions, lie at the interface between emotion and cognition and form part of the mesolimbic fronto-striatal dopamine pathway modulating reward processing (
53), reward-based decision making, and motivation control (
54). Recent meta-analyses and fMRI reviews of decision making show that both structures are crucial for the integration of affective and reward information into cognitive processes governing decision making (
55,
56), such as reappraisal (
56,
57), reward-based decision making (
54,
58,
59), reward processing (
60), reinforcement learning (
61,
62), and intertemporal choice (
54,
55,
63). The dysfunction finding is parallel to two recent whole-brain structural MRI meta-analysis findings of reduced gray matter in the anterior cingulate in youths with conduct problems, and in the dorsomedial and frontopolar prefrontal cortices in youths with antisocial behavior (
15,
64). This abnormality in decision making mediated by the dorsomedial and prefrontal cortices and in the reward-processing region of the ventral caudate may represent the neural underpinning for evidence that perturbed reward-based decision making is key to conduct disorder with and without psychopathic traits and is more common than perturbed empathy or threat sensitivity (
65). This abnormality may contribute to the maladaptive impulsive-aggressive, norm-violating behaviors observed in this population (
5), possibly due to increased frustration resulting from poor decisions that lead to reactive aggression (
66). Male gender was associated with more severely decreased function of the dorsal anterior cingulate. However, this finding must be interpreted with caution because males made up more than 50% of most study populations. A caveat is that the majority of fMRI studies included in this meta-analysis tested hot executive functions, given consistent neurocognitive impairments (
4,
5,
8,
65), which likely biased the findings. Future meta-analyses of a larger number of fMRI studies of emotion processing may reveal more abnormalities in the orbitofrontal and limbic regions.
The sub-meta-analysis of cool executive function revealed right superior and middle temporal dysfunction in the disruptive/conduct problems group. The temporal lobes have been suggested to be dysfunctional in neurobiological theories of conduct disorder and psychopathy (
12,
67) because they are among the most consistently observed structural deficit regions (
9,
14,
15,
64,
68). The temporal lobes form part of the paralimbic motivation system, and together with the amygdala, they mediate stimulus-reinforcement learning (
69); hence, temporal lobe hypoactivity may reflect insufficient motivation (
4). Alternatively, superior temporal regions have also been associated with attention functions (
70,
71) that are affected in the disorder (
3,
4).
The decreased activation of the right dorsolateral prefrontal region during emotion processing also suggests poor frontal top-down cognitive control over emotion processing, a key functional role of this region (
57,
72), while reduced function of the left temporal pole may reflect impaired socio-emotional processes (
73). Interestingly, older patients had more dorsolateral prefrontal dysfunction, which may suggest progressive age-related impairments. However, the reliability analysis showed that the temporal dysfunction was found only in two fMRI studies (
20,
30), while dorsolateral prefrontal dysfunction was found only in the largest study (
30). Unexpectedly, we did not observe abnormalities in limbic regions, such as the amygdala, during emotion processing. The amygdala is a relatively small region and is rarely observed in whole-brain studies (e.g.,
36,
40); it is examined mostly in region-of-interest fMRI studies (
24,
27–
29,
32). Furthermore, during negative emotions, amygdala activation has been found to be decreased in conduct disorder with psychopathic traits but increased in conduct disorder without psychopathic traits (
66), which may have resulted in negative findings because most included studies did not screen out individuals with psychopathic traits.
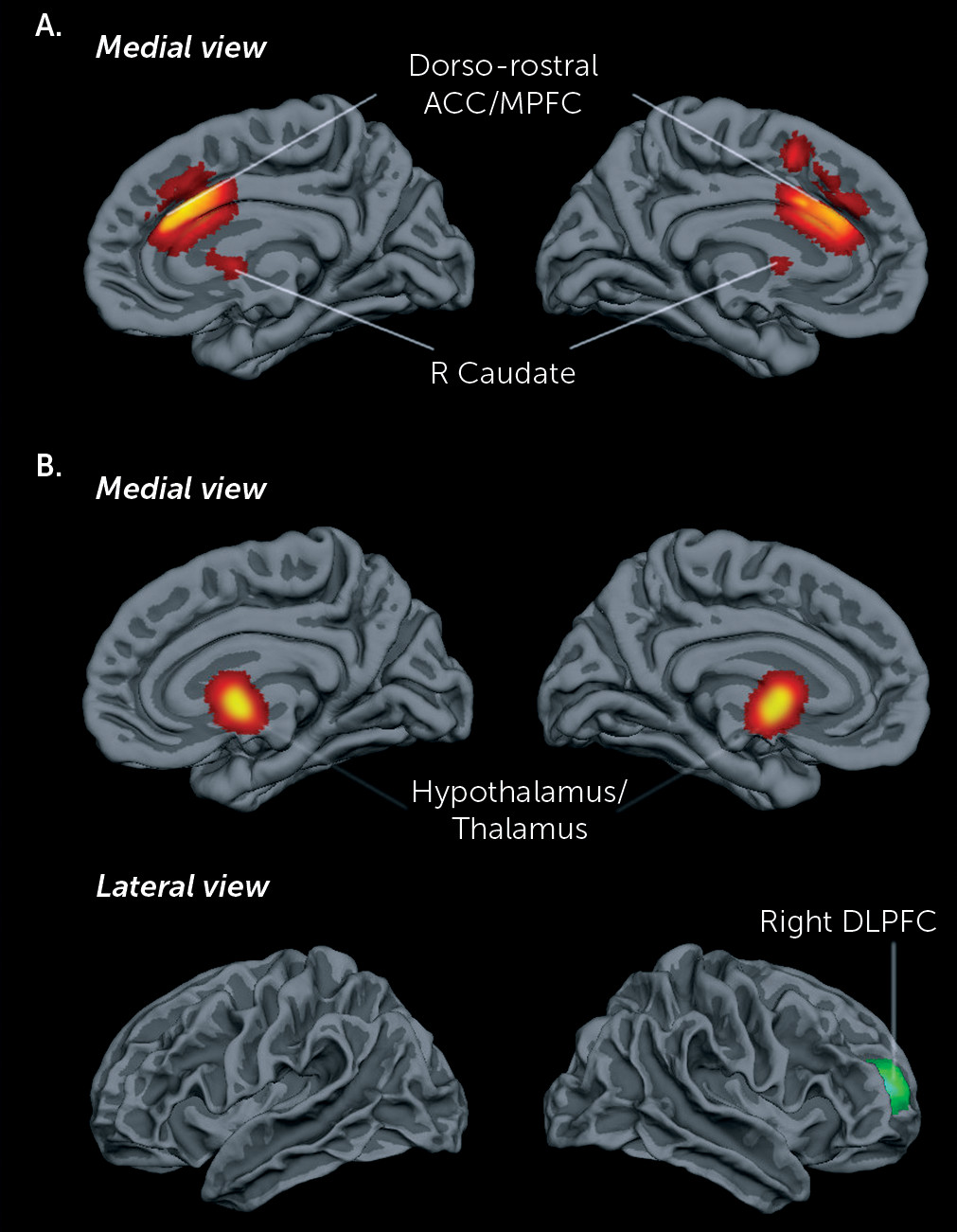
The subgroup meta-analysis findings in youths with disruptive/conduct problems and psychopathic traits differed from those in the whole group, in line with evidence for different neurological etiological mechanisms in conduct disorder with and without psychopathic traits (
44,
65,
66,
74). Thus, the functional deficits in this subgroup were in the ventromedial prefrontal-limbic regions known to be involved in reward and decision making and in areas of affective reactivity, especially to negative emotions, such as the hypothalamus and thalamus (
75,
76). Hypothalamus hypoactivity is consistent with evidence for abnormal reactivity in the hypothalamic-pituitary-adrenal neuroendocrine system and with reduced levels of cortisol in this group (
77,
78), and these levels furthermore are correlated with psychopathic traits (
79,
80). The underfunctioning in the ventromedial prefrontal-hypothalamic regions, both closely interconnected with the amygdala, may play a role in the psychopathic symptoms of reduced affect, such as reduced responsiveness to threat and distress cues, lack of empathy, low anxiety levels, and guilt (
65,
66,
81,
82). The ventral striatum is a key region of reward and loss processing and is thought to be at the core of psychopathic traits (
83–
85). The deficit findings are in line with Blair’s psychopathy model (
65,
66) of ventromedial prefrontal, amygdala, hypothalamus, and striatal abnormalities, with the exception that we found no amygdala underactivation. As discussed above, this may be due to the use of whole-brain fMRI analyses and a prevalence of fMRI studies of reward-based decision making. Overactivation of the rostral dorsolateral prefrontal cortex and the dorsal caudate in the disruptive/conduct problems group with psychopathic traits is in line with findings of abnormally increased caudate volumes in psychopathic adults and violent offenders (
86,
87); with higher structural connectivity in cingulo-fronto-striatal tracts in adolescent arrestees, correlated with grandiose-manipulative traits (
88); and with correlations between dorsolateral prefrontal hyperactivity and psychopathic traits (
89). The rostral dorsolateral prefrontal cortex and caudate are involved in planning (
90,
91), and enhanced activity in these regions is in line with neurocognitive studies showing no deficits in executive functions, or even superior executive functions, such as in planning, set-shifting, and language abilities (
92–
95), and it matches the defining features of proactive, planned, and goal-directed aggression (as opposed to frustration- or threat-induced reactive aggression in those without psychopathic traits) (
96), as well as the ability to manipulate, cheat, and con. A dysfunctional affect and a hyperfunctional executive control system in disruptive groups with psychopathic traits provide neurofunctional support for behavioral theories of good executive functioning in the presence of dampened affect. Thus, it has been suggested that a hypoactive bottom-up affective system (reflecting reduced affective reactivity and lower anxiety), together with good top-down executive control over emotions, may lead to less emotional interference with cognitive functions, explaining superior performance in psychopathy (
92–
95).
However, the subgroup meta-analysis on disruptive/conduct problems with psychopathic traits should be treated with caution, as studies were heterogeneous in methods, informants, and cutoff scores for psychopathic traits. Future studies need to clearly distinguish groups with disruptive behavior disorder with and without psychopathic traits based on internationally agreed-upon, age-normalized, standardized measures from multiple informants to establish the neurofunctional underpinnings of both subtypes (
97–
99).
The meta-regression analyses showed that ADHD comorbidity, age, or medication had no effect on dysfunctions, suggesting that they are specific to disruptive behavior disorder. Despite evidence of dorsal anterior cingulate underfunctioning in ADHD during executive functions (
4,
47), comparison between ADHD comorbid and noncomorbid with conduct disorder showed that dorsal anterior cingulate underactivation was specific to conduct disorder (
4,
16). In addition, rostro-dorsal anterior cingulate dysfunction in conduct disorder in fMRI studies of emotion processing remained when ADHD was controlled for (
24) and correlated specifically with conduct disorder symptoms and aggressive behavior (
24,
36,
100). Structural analyses also found anterior cingulate volume to be associated with disruptive behavior disorder when ADHD was included as a covariate (
101). Hence, findings of underactivation in the rostro-dorsal anterior cingulate in ADHD may be associated with commonly co-occurring antisocial features (
4). Meta-analytic fMRI evidence in ADHD also suggests more prominently lateral, rather than medial, frontal underactivation during executive functions (
46,
47). Alternatively, reward-based decision making, which is also impaired in ADHD (
4), even if it is mostly accounted for by antisocial behaviors in dimensional analyses (
8), may be a transdiagnostic endophenotype of both ADHD and disruptive behavior disorders, with a common underlying neural substrate in the dorsomedial prefrontal cortex. However, ventral striatum underactivation is also a consistent meta-analytic finding in ADHD during reward anticipation (
102), based on region-of-interest studies. This dysfunction has not been observed in whole-brain meta-analyses of ADHD, which could explain the lack of association with ADHD comorbidity. Alternatively, ventral striatum dysfunction in ADHD may be associated with comorbidity with conduct disorder, which is rarely excluded in ADHD fMRI studies.
This study has a number of limitations inherent to all meta-analyses. First, meta-analyses based on peak and effect size use data from published studies rather than raw statistical brain maps, increasing the likelihood of having less accurate results (
49). Second, different studies used different statistical thresholds. Third, while the voxel-wise meta-analytic method provided good control of false positive results, false negative results are more difficult to avoid, making results more conservative (
49). Fourth, although substance abuse is common among youths with disruptive/conduct problems and has an important effect on brain structure and function (
103,
104), many studies including youths with substance use disorder comorbidity did not report case numbers (
17,
20,
27,
32,
37,
44,
100), hampering our ability to examine its effect. It is also likely that the neurofunctional substrates of patients with pure oppositional defiant disorder differ from those of patients with pure conduct disorder, and future studies should address this heterogeneity. Fifth, studies have suggested differences between early- and late-onset disruptive behavior disorders (
10,
30), but there was insufficient information to conduct subtype meta-analyses. Sixth, mean age ranged only from 11.9 years to 17.7 years, and therefore the age-based meta-regression analysis should be interpreted with caution. Seventh, seed-based
d mapping software does not directly take into account the reported cluster size, which could improve the re-creation of effect size maps. However, cluster size is indirectly accounted for through the use of cluster local peaks and the fact that cluster size depends on the height of the peaks and the local covariance between neighboring voxels. Lastly, the sub-meta-analysis of cool executive functions was relatively underpowered with only eight data sets, and 50% of the studies came from the same research group using the same 13–14 subjects, which renders the subdomain meta-analysis findings unrepresentative. Further research on cool executive functions in groups with disruptive behavior disorder or conduct problems is needed.
In summary, to our knowledge this is the first meta-analysis of fMRI studies of deficits in youths with disruptive behavior disorder or conduct problems. The meta-analysis shows that the core dysfunction in this population lies in the rostro-dorsal and medial fronto-cingulate regions that exert top-down control over interconnected limbic motivation systems (such as the ventral caudate, which is also underactivated) and that underlie reward-based decision making, which is typically compromised in the disorder. Psychopathic traits in the disorder are more prominently associated with ventromedial frontal-hypothalamic-limbic underfunctioning and dorsolateral prefrontal-striatal hyperfunctioning, which presumably reflect poor empathy and affect reactivity together with and perhaps caused by enhanced dorsolateral prefrontal-striatal top-down control. Finding dissociated neuro-functional correlates in the disruptive-behavior groups with and without psychopathic traits adds to increasing evidence for different underlying neurobiology and supports the utility of the DSM-5 callous-unemotional specifier in the classification of youths with conduct disorder. The meta-analysis findings provide potential targets for neurotherapeutic and pharmacological interventions.