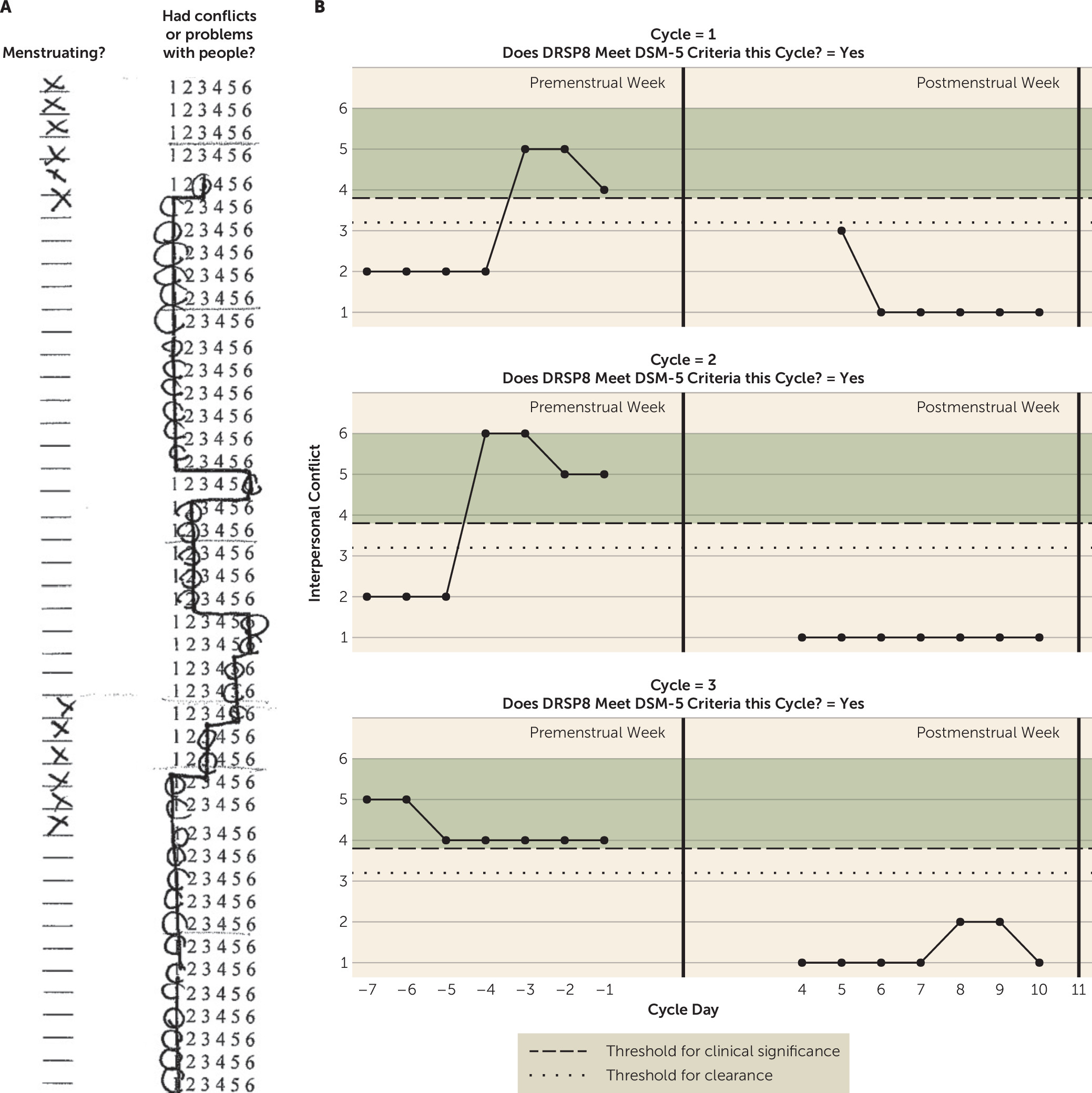
Premenstrual dysphoric disorder (PMDD), characterized by the emergence of emotional symptoms in the luteal phase of the menstrual cycle, causes severe distress and impairment among the estimated 3%−8% of women who meet DSM-5 criteria for the disorder (
1,
2). Another 10%−11% of women show evidence of a menstrually related mood disorder (MRMD) that causes distress and impairment sufficient to warrant treatment despite failure to meet full DSM-5 criteria for PMDD (
2). Because of the poor prospective validity of retrospectively reported premenstrual symptoms, valid diagnosis requires evaluation of prospective daily symptom ratings (
3). In research settings, diagnosis is often made by visual inspection of daily symptom ratings (
4) (
Figure 1, panel A). However, laboratories differ in the specific manner in which daily ratings are translated into diagnostic decisions (
5,
6), and the complex, multilevel nature of the diagnosis suggests a high risk of diagnostician error. These issues motivated development of the Carolina Premenstrual Assessment Scoring System (C-PASS), a standardized, computerized procedure for the reliable prospective diagnosis of DSM-5 PMDD.
DSM-5 PMDD is multifaceted and multilevel, requiring that many conditions be met (content, cyclicity, severity, and chronicity) at various levels (symptoms, cycles, women). DSM-5 symptoms and their overlap with the items of the Daily Record of Severity of Problems (DRSP) (
7), the most widely used daily symptom scale, are listed in
Table 1.
Table 2 outlines our conceptualization of DSM-5 diagnostic dimensions: 1) the
content dimension, which refers to the nature and number of symptoms; five symptoms must be present, of which one must be a core emotional symptom; 2) the
cyclicity dimension, referring to both relative premenstrual elevation (“premenstrual change”) and absolute postmenstrual clearance of symptoms, which describes the required premenstrual onset and postmenstrual offset of symptoms in the perimenstrual time frame (
8); 3) the
clinical significance dimension, which dictates that symptoms must be of sufficient absolute premenstrual severity and premenstrual duration as to cause clinically significant distress or impairment; and 4) the
chronicity dimension, which requires that symptoms be present in the majority of months.
The DRSP (
7) measures all 11 DSM-5 PMDD symptoms. Women rate daily symptoms on a 6-point scale from 1 (not at all) to 6 (extreme). This allows for evaluation of the symptom dimensions described above; however, DSM-5 does not give numeric thresholds for most dimensions, leading to variability in thresholds used across laboratories. Although the field has made some strides in standardizing diagnosis (
9), at least two key inconsistencies remain. First, the DSM-5 requirements of “severe” premenstrual symptoms (absolute severity) and “minimal or absent” postmenstrual symptoms (absolute clearance) are subjective, and different studies set the threshold for clinical significance of symptoms at different rating levels on the DRSP (
4). The developers of the DRSP suggest that the most liberal acceptable delineation of “clinically significant” symptoms would be a rating of 4 (“moderate”) or higher (
7). To reduce the risk of diagnosing normal affective experiences as a mental disorder (
5,
6,
10), we recommend that this cutoff of 4 be implemented consistently as the threshold for absolute severity (premenstrual symptoms must reach a rating of 4) and absolute symptom clearance (postmenstrual symptoms must not exceed a rating of 3). Second, although 30% premenstrual symptom elevation (or premenstrual “change”) is generally used as a threshold for significant symptom cyclicity (
8,
11), at least five different methods have been used to calculate this variable (
Table 3) (
4,
9,
12–
15). Therefore, in the present study, we begin by examining the combined influence of both differing calculation methods and differing thresholds on diagnostic prevalence in our sample.
Even with validated numeric thresholds, many factors may limit the reliability of PMDD diagnoses made using visual inspection, making computerized diagnosis preferable. First, although it is possible to evaluate many of the diagnostic dimensions by simple visual inspection of daily ratings, premenstrual symptom elevation relative to one’s postmenstrual (follicular) symptoms—which is the most discriminating feature of PMDD (
11)—cannot be readily determined through visual inspection, and therefore must be calculated for each symptom in each cycle. Second, validated numeric criteria have limited clinical utility if a clinician must calculate and concatenate the diagnostic dimensions manually at symptom, cycle, and person levels across 1,700 daily ratings (i.e., 3 months). Third, visual inspection of ratings across the entire cycle requires that the diagnostician ignore a great deal of distracting information that is not central to the diagnosis of PMDD. Finally, common errors of clinical judgment, such as the tendency to ignore base rates (which in this case are low; 3%−8% with PMDD and an additional 10%−11% with non-PMDD MRMD [
2,
16]) and to assign too much importance to readily available information (e.g., absolute premenstrual symptom severity versus the more complicated relative premenstrual symptom elevation), may introduce diagnostic error (
10–
12). Therefore, although valid diagnosis of PMDD is possible using simple visual inspection (
7), poor reliability of this approach is likely due to busy clinician schedules and sources of unconscious error. It is this state of affairs that motivated our development of a computerized approach (
13) to making the complex diagnosis of PMDD.
Here, we describe development of the C-PASS in a sample of 200 women with self-diagnosed PMDD. We had four goals. First, by providing this standardized scoring system, we aimed to promote the reliability—and, by extension, the construct validity—of the PMDD diagnosis by eliminating diagnostician variability and error. Given the important sociocultural concerns raised around the DSM-5 diagnosis, we believe that a move toward diagnostic specificity and reliability is critical (
5,
6,
10). Second, C-PASS data output (from the SAS macro) provides dimensional variables for each woman, with the goal of promoting the dimensional (as opposed to the categorical) study of premenstrual dysphoria. Third, C-PASS visual output (from the SAS macro) maximizes attention to central diagnostic information (
Figure 1, panel B) with the goal of integrating the benefits of visual inspection with the reliability of computerization. Fourth, the C-PASS aims to improve the clarity of pathophysiological studies of PMDD by permitting more homogeneous clinical samples.
Method
The C-PASS Diagnostic Method
The manual worksheet and the SAS and Excel macros for the C-PASS are available at
https://www.med.unc.edu/psych/wmd/resources/clinicians-researchers/C-PASS (the worksheet is also provided in the
data supplement that accompanies the online edition of this article). The SAS macro was developed using a double-coding technique by authors T.A.E.M. and J.L.J. Because DSM-5 PMDD is defined as a marked on-off pattern occurring in the perimenstrual time frame, the C-PASS defines a “cycle” as the premenstrual week of one menstrual cycle (defined as days −7 to −1, where −1 is the day before menstrual onset) and the postmenstrual week of the following menstrual cycle (defined as the 7 days following average menstrual offset: days 4 to 10, where day 1 represents menstrual onset). The rationale for comparing the premenstrual week of one menstrual cycle to the subsequent postmenstrual week of the next cycle is to establish the “switch off” of symptoms, as it is critical to demonstrate that premenstrual symptoms do not persist into the follicular phase. The C-PASS also requires that at least three out of seven ratings be available in each of the two weeks, and it requires two cycles of ratings (i.e., two perimenstrual frames).
The diagnostic process begins by characterizing each DRSP item in each cycle using the four diagnostic dimensions described in
Table 2. First, the symptom must show adequate relative premenstrual symptom elevation (i.e., elevated relative to the following postmenstrual phase), defined as a percent symptom difference of ≥30% between the mean rating for the premenstrual week and the mean rating for the postmenstrual week (see
Table 3). Second, the symptom must show absolute clearance, defined as a maximum postmenstrual week rating ≤3 (“mild”). Third, the symptom must show sufficient absolute severity, defined as a premenstrual week maximum rating ≥4 (“moderate”). Finally, these clinically significant symptoms must show sufficient premenstrual duration, defined as at least 2 days in the premenstrual week in which the symptom is rated ≥4. If all four of these criteria are met, the symptom meets criteria for the symptom pattern described in DSM-5 PMDD in the present cycle. Each symptom in each cycle is evaluated separately in this manner.
While the DSM-IV research diagnosis of PMDD required premenstrual impairment, the DSM-5 criteria do not require impairment for diagnosis. Therefore, the interference items on the DRSP are not included in the C-PASS diagnostic process, although the SAS macro provides information about the cyclicity and clinical significance of life interference items from the DRSP as additional outcomes.
Next, MRMD (which subsumes both PMDD and non-PMDD MRMD) or PMDD diagnosis is made at the cycle level by counting the number of DSM-5 symptoms that meet the four criteria above (see
Table 2). If multiple DRSP items measuring the same DSM-5 symptom (e.g., “felt angry, irritable” and “had conflicts or problems with people”) meet the four criteria described above, just one DSM-5 symptom is counted. For a cycle to meet DSM-5 criteria for PMDD, at least five symptoms must meet criteria in that cycle, and one of those symptoms must be a core emotional symptom (DRSP items 1 through 8; see
Table 1). To meet the less stringent criteria for MRMD (
8), a cycle must meet criteria on at least one core emotional symptom, but there is no requirement of a higher total number of symptoms.
Next, MRMD or PMDD diagnosis is made at the person level by counting the number of cycles that meet MRMD and PMDD criteria. If a woman meets PMDD criteria in at least two cycles, the C-PASS makes a diagnosis of PMDD. If a woman meets MRMD criteria in at least two cycles, the C-PASS makes a diagnosis of MRMD. Of note, it is possible for a woman who has provided ratings for the minimum of two cycles to meet criteria for PMDD in one cycle while only meeting criteria for MRMD in the other cycle. Although this was not possible in the present study, users of this diagnostic system may wish to gather an additional cycle of ratings from such women in order to further assess for PMDD.
These diagnostic decisions can be made using the SAS or Excel macros or manually using the worksheet. The SAS macro additionally provides 1) a visual representation of perimenstrual symptom patterns for each DRSP item across as many cycles as provided, labeled with the diagnostic decision for that item in that cycle (as shown in
Figure 1, panel B), and 2) a data set with between-person summary variables for use in further analyses, including average number of DSM-5 symptoms met per cycle, average percent premenstrual symptom elevation of each DRSP item across all cycles, average maximum premenstrual severity of each DRSP item across all cycles, and average number of severe (rated ≥4) premenstrual days of each DRSP item across all cycles.
Participants, Procedure, and Materials
Between 2009 and 2015, naturally cycling women ages 18–47 years (mean=32.7 years, SD=8.21) with regular cycles (21–35 days) were recruited through flyers and e-mails seeking women with premenstrual emotional symptoms consistent with DSM-5 PMDD. Women were excluded if they had a chronic medical disorder, a history of mania, substance dependence, or psychosis, or any current DSM-IV axis I diagnosis or if they used antidepressants, benzodiazepines, antipsychotics, or hormonal preparations. Participants were not paid. At a baseline visit, participants reported their medical and medication history and completed the Structured Clinical Interview for DSM-IV Axis I Disorders (
17). Participants also retrospectively reported the degree of premenstrual increase in each of 21 symptoms (
18) on a 4-point Likert scale from 1 (no change) to 4 (severe change) (Cronbach’s alpha=0.91). A total of 267 eligible women then completed prospective assessment.
The prospective assessment included two to four cycles of daily DRSP ratings, along with daily reports of external events participants believed to have affected their mood. Ratings for days on which participants reported the occurrence of a severe stressor not caused by symptoms were coded as missing. Participants mailed in forms weekly. In the final sample, 200 women provided ratings for at least two cycles. Eighty-five percent of the women who dropped out after one cycle had not met C-PASS PMDD criteria in the first cycle. Among women who provided ratings for two or more cycles, missing days were minimal (3.4%), and only 1% of daily data were coded as missing because of external events. Expert diagnoses (by D.R.R.) of MRMD made before the development of the C-PASS (on the basis of identical data) were available for the majority of our sample (N=193; 96.5%). Because the DRSP’s summed total score demonstrates inadequate reliability of change (
19), descriptive statistics for single items are considered.
Results
At least five equations have been used in the literature to calculate relative premenstrual symptom elevation, and several thresholds for diagnosis have been proposed (30%, 50%, and 75%). With premenstrual week average minus postmenstrual week average as the constant numerator, the five calculation methods differ in the denominator used: 1) postmenstrual (follicular) mean (denominator: postmenstrual week average), varying by symptom and cycle; 2) range of scale used across all ratings (denominator: woman’s maximum rating minus one), varying by woman; 3) full-scale range (demonimator: fixed at five); 4) premenstrual mean (denominator: premenstrual week mean), varying by symptom and cycle; and 5) standard deviation (denominator: standard deviation of this symptom in this cycle for this woman), varying by symptom and cycle. Of note, the standard deviation method utilizes a constant one-standard-deviation threshold. In
Table 3, we examine the combined impact of these five calculation methods and three thresholds on diagnostic prevalence.
Calculation method and threshold used to define significant relative premenstrual symptom elevation had a large impact on diagnostic prevalence (see
Table 3). The follicular mean method consistently proved to be the most liberal, resulting in much higher average relative premenstrual symptom elevation values (p<0.001 for all comparisons) and the highest prevalence rates. The premenstrual mean and standard deviation methods appeared slightly more conservative, while the range-of-scale-used and full-scale methods appeared most conservative. Increasing percentage thresholds reduced diagnostic prevalence, particularly when using full-range and range-of-scale-used methods. For the C-PASS protocol, we selected the range-of-scale-used method paired with a 30% threshold because this particular combination produced the highest agreement with expert clinical diagnosis of MRMD. This combination also produced prevalence rates that were reasonable in light of epidemiological studies (
9), assuming that rates of diagnosis should be somewhat higher in this sample of women seeking to participate in a study of premenstrual affective symptoms. The use of the range-of-scale-used method also maximizes the validity of the results in women who may not use the full DRSP response scale. In the context of the range-of-scale-used method, a threshold of 30% produced 94.5% diagnostic agreement with expert clinical diagnosis, whereas a 50% threshold produced just 34% agreement and a 75% threshold produced just 12% agreement.
Once the protocol was finalized, we used the C-PASS to identify three diagnostic groups: no diagnosis (N=116, 58%), non-PMDD MRMD (N=46, 23%), and DSM-5 PMDD (N=38, 19%).
Table 4 presents descriptive statistics for diagnostic dimensions by group.
Criterion Validity: Comparing C-PASS Diagnosis With Expert Diagnosis
Comparison of C-PASS decisions (MRMD versus no MRMD) with expert diagnosis revealed 94.5% agreement (11 disagreements). Inspection of ratings and clinical notes revealed three reasons for disagreement, each of which is instructive as to either the usefulness of the C-PASS or ways to improve it.
In four cases, women who were diagnosed as having MRMD by the C-PASS were not diagnosed with MRMD by expert clinician because of persistent background symptoms. Inspection of the raw data revealed that the C-PASS had identified repeating cyclical patterns of anxiety (in two women) or interpersonal rejection sensitivity and anger (in two women) that occurred in the context of other, more persistent symptoms. DSM-5 does allow a diagnosis to be made in such cases, as long as this pattern of symptoms does not represent an exacerbation of an underlying mood disorder, which was exclusionary in this study. Given 1) the clear evidence of repeating premenstrual elevations and postmenstrual declines on these symptoms and 2) the absence of DSM-IV axis I disorders in this sample, we believe that the C-PASS decision for these women is accurate.
In three other cases, women with insufficient premenstrual symptom severity (symptoms failing to reach a rating of 4 [moderate] in the premenstrual phase) or insufficient duration of severe symptoms (less than 2 premenstrual days of at least moderate symptoms) were diagnosed with MRMD by expert clinician but were not diagnosed by the C-PASS. Although these women showed genuine menstrual cycle entrainment of symptoms, they failed to meet the a priori definition of a clinically significant mental disorder, and we believe that the C-PASS decision not to diagnose these women is accurate.
In the remaining four cases, women with “late” menstrual clearance of symptoms (symptoms persisting through the end of menses) were accurately diagnosed with MRMD by expert clinician, whereas the C-PASS, which evaluates symptom clearance during days 4 to 10, failed to diagnose them because it judged that the symptoms had not cleared adequately. We believe that the expert clinician was correct in these cases to allow for more individual variability in the timing of postmenstrual symptom clearance, and this provides an important area for improving the C-PASS. In a future study (for which data collection is under way), we will evaluate differences between diagnosis made based on standardized days 4–10 and diagnosis made based on an individualized last day of menses plus 7 days. Although the latter may seem the intuitive choice, the former may have the benefit of greater biological validity, as the start of menstrual bleeding is a less ambiguous stimulus to self-report than the end of menses. In sum, we believe that the C-PASS mistakenly failed to diagnose just four women with MRMD (2% of the sample).
Comparison of Retrospective Premenstrual Symptoms to C-PASS Diagnosis
Consistent with previous reports (
18), logistic regression revealed that retrospectively reported premenstrual symptom change was a significant but weak predictor of both C-PASS MRMD diagnosis (standardized B=0.038, SE=0.011; χ
2=11.46, p<0.001; odds ratio for a one-standard-deviation increase in retrospective symptoms, 1.03, 95% CI=1.01–1.06) and C-PASS PMDD diagnosis (standardized B=0.061, SE=0.016; χ
2=14.01, p<0.001; odds ratio for a one-standard-deviation increase in retrospective symptoms, 1.06, 95% CI=1.03–1.09). Given this poor predictive validity of retrospectively reported premenstrual symptoms, attempts to identify a reasonably sensitive and specific cutoff value for the prediction of C-PASS diagnoses were unsuccessful (for MRMD diagnosis, area under the curve [AUC]=0.63, 95% CI=0.56–0.70; for PMDD diagnosis, AUC=0.70, 95% CI=0.62–0.78).
Discussion
Despite evidence for the existence and burden of PMDD (
9), inconsistent diagnostic practices compromise the construct validity of the disorder (
10), undermine accurate clinical diagnosis (
14), and threaten the clarity of efforts to characterize the pathophysiology of the disorder. In an effort to hasten and streamline the translation of the DSM-5 PMDD criteria into standardized terms compatible with existing research practices, this study presents the C-PASS, a scoring system for prospective ratings on the DRSP that can be used either manually or with macro programs for SAS and Excel.
This study also draws attention to and resolves an egregious mathematical inconsistency in the literature: The use of at least five different equations for calculating the degree of premenstrual symptom elevation relative to baseline (often referred to as “premenstrual change”) has introduced unacceptable between-laboratory and between-clinic variability in the meanings of MRMD and PMDD. In light of the present validity findings, we recommend that the range-of-scale-used method (
Table 3) be utilized in combination with a 30% threshold. Whatever methods are used, both calculation equations and thresholds should always be explicitly described in studies of MRMD and PMDD. This represents a crucial step toward construct validity and replicability of findings.
The present work holds the potential to increase the reliability of PMDD diagnosis; however, additional work should further examine the validity of the diagnostic thresholds used in the C-PASS, especially the number of symptoms per cycle that best defines a group of women in need of diagnosis and treatment. Of note, the dimensions of PMDD diagnosis were normally distributed; ultimately, PMDD may be best conceptualized in a continuous, dimensional manner that could be described more precisely in future editions of DSM (“unisymptom” versus “multisymptom”; or “with rapid offset” versus “with gradual offset”). Future research must also determine whether MRMD and PMDD arise from normally distributed risk processes related to those described in the Research Diagnostic Criteria (
15,
20) framework, or whether there are more categorical pathophysiological processes at play.
Limitations and Future Directions
Several limitations of this study are noteworthy. First, because the C-PASS was designed to identify premenstrual symptoms of a clinically significant nature, it may fail to identify women who demonstrate significant menstrual-cycle entrainment of symptoms that are not premenstrual (e.g., affective symptoms at midcycle [
21]), or those whose symptoms do not show sufficient absolute severity or clearance. Second, because the C-PASS seeks to balance efficiency with reliability, it does not rule out the possibility of significant late follicular symptoms; however, if significant late follicular symptoms are present, this may signal the need for differential diagnosis. Third, the C-PASS was developed for the DRSP, given that this scale assesses all DSM-5 content; however, other useful rating scales for PMDD exist (e.g., the Daily Symptom Record [
22]). Limitations of the DRSP, such as scale sensitivity (i.e., relative to a 100 mm scale) and unbalanced content coverage, should be addressed. Fourth, the C-PASS may fail to accurately diagnose some women with late symptom clearance; future work should determine whether self-reported menstrual offset is sufficiently accurate to permit the use of personalized postmenstrual weeks for each woman. Additional validation work must demonstrate that the methods and thresholds selected here are calibrated to ensure that only women who need treatment are diagnosed with PMDD. Finally, women seeking treatment for PMDD in clinical settings may prove to be qualitatively different from women in research settings.
Research Applications
The C-PASS has myriad applications in research. In the context of clinical research, the use of a standardized, reliable system of diagnosis would ensure shared diagnostic meaning across laboratories, and it would ensure homogeneous samples. The C-PASS also allows for dimensional characterization of symptoms across individuals and samples according to central dimensions of PMDD diagnosis (see
Table 2), which may allow for the eventual identification of meaningful differences, if any, between women with PMDD and those with other MRMDs. Further development of this system may allow for the identification of distinct subgroups with differing pathophysiology (
23). Finally, it should be noted that the C-PASS can also be used to identify cycles meeting criteria for PMDD, a feature that could be useful for defining treatment response in the context of clinical trials.
Clinical Implications
There are currently no reliable and valid diagnostic procedures for PMDD that are feasible for widespread clinical application. Given the time involved in prospective assessment, nearly 90% of clinicians who treat PMDD rely on patient retrospective self-report, which is known to be prone to false positives, to make diagnoses (
14). This is troubling when considered in tandem with the present evidence that 1) there is a relatively low prevalence of true PMDD even among women seeking assessment for premenstrual symptoms, and 2) variability on retrospective self-report of premenstrual symptoms does not provide substantial information about whether a standardized, prospective diagnosis of PMDD is present. Rigorous experimental (
24) and longitudinal (
16) studies have established the biological validity of PMDD, and treatments are available; however, assessments that combine validity, reliability, and efficiency need to be developed so that women with the disorder can receive treatment, and women without the disorder can search for alternative causes of their symptoms. Standardization of research diagnoses through the C-PASS represents an initial step toward development of efficient and reliable clinical tools. The current C-PASS materials may be immediately useful clinically; however, additional development is needed to digitize data collection and streamline the diagnostic process for clinical application.