Major depressive disorder, the second leading cause of disability globally (
1,
2), accounts for one-tenth of all years lived with disability (
3). Reduced work productivity accounts for over 80% of the financial costs attributed to depression (
4), as depressed patients have substantial work productivity impairments (
5,
6). Presenteeism (reduced productivity while at work) and absenteeism (absence from work) have been associated with the loss of 18.2–46.8 and 7.8–8.7 workdays per worker per year, respectively (
7,
8). Among baseline clinical and sociodeomographic variables, depressive symptom severity was the strongest predictor of work productivity impairment after a year (
9) in a community-based cohort (
10). However, several other factors have been shown to be associated with poor work productivity (e.g., part-time employment status [
6], fair or poor general health [
6], history of suicide attempt [
5], anxious features, and earlier age at onset of depression [
5]). Impaired cognitive function has also been linked to disrupted work productivity in depressed patients (
11,
12).
With effective antidepressant treatments, work productivity improves (
5,
13–
15), and this improvement is predicted by baseline levels of depression symptoms and work productivity (
13) along with other clinical (e.g., recurrent episodes, melancholic or atypical features) and demographic (e.g., education level) factors (
5). Outside of reduction in depression symptom severity, changes in cognitive function may be an important factor in improvement of work productivity. Recent data from the International Mood Disorders Collaborative Project suggest that cognitive function explains variability in workplace function to a greater extent than depression symptom severity (
16). Importantly, both cognitive and workplace impairments are often still noted even with achievement of remission of depression symptoms (
17).
The long-term deleterious effects of greater depression severity on work (
9,
18–
20) are well known. As a baseline factor, being employed (
5,
6,
21,
22) has been shown to predict better clinical outcomes. However, whether changes in work productivity affect the typically chronic or recurrent course (
23–
25) of depression is unknown. Our knowledge of the relationship between depressive symptoms and work productivity is also limited by the fact that most studies of work productivity improvement during antidepressant treatment have measured changes only between baseline and posttreatment (
5,
13–
15). Multiple assessments over the course of treatment as used in the present study would clarify better how depressive symptoms relate to work productivity improvement.
The present study, a secondary analysis, tested the hypothesis that early improvement in work productivity would be associated with better long-term outcomes. Using data from the Combining Medications to Enhance Depression Outcomes (CO-MED) trial (
26), a multisite single-blind randomized controlled trial of outpatients with chronic and/or recurrent nonpsychotic depression, we repeatedly measured and evaluated changes in work productivity associated with antidepressant treatment. The CO-MED trial included both an acute phase of 12 weeks (3 months) and a continuation phase of an additional 16 weeks (total 7 months) for patients with a clinical response, thereby allowing for evaluation of the relationship between work productivity and antidepressant response over the course of 7 months of treatment (
26). We addressed this issue by identifying subgroups of trial participants based on work productivity changes during the first 6 weeks of acute-phase treatment and estimated the association of these groups with depressive symptom status/improvement during the remaining 6 weeks of acute-phase treatment and the ensuing 16 weeks of continuation-phase treatment.
Method
Study Overview and Participants
The CO-MED trial was approved by the institutional review boards at the University of Texas Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, and each participating regional center and clinical site, and it was monitored by an independent data safety and monitoring board. All participants provided written informed consent prior to completing study procedures.
Details of the study design, measurements, and primary outcomes are available elsewhere (
26). From six primary and nine psychiatric care sites, CO-MED enrolled 665 participants with nonpsychotic chronic (current episode exceeded 2 years) or recurrent depression with a current episode ≥2 months and a 17-item Hamilton Depression Rating Scale (HAM-D-17) score ≥16.
Participants, stratified by clinical sites, were assigned in a 1:1:1 ratio to 1) escitalopram plus placebo (selective serotonin reuptake inhibitor [SSRI] monotherapy), 2) sustained-release bupropion plus escitalopram (bupropion combination), or 3) extended-release venlafaxine plus mirtazapine (venlafaxine combination). Measurement-based care (
27) was implemented by study physicians at each visit to tailor antidepressant dosage adjustments for each individual participant based on scores on the Quick Inventory of Depressive Symptomatology-Clinician-Rated version (QIDS-C) (
28) and the Frequency, Intensity, and Burden of Side Effects Rating scale (
29).
Assessments
The measures below were obtained at baseline and each subsequent study visit of the acute phase (weeks 1, 2, 4, 6, 8, 10, and 12) and the continuation phase (weeks 16, 20, 24, and 28).
QIDS-C and QIDS-Self-Report (QIDS-SR).
The total score for the QIDS-C and QIDS-SR (range of 0–27) is based on the nine criteria symptom domains out of the 16 items, each of which is scored from 0 to 3 (
28). In a previous report, the Pearson product-moment correlation coefficient between QIDS-SR and HAM-D-17 was 0.86, and between QIDS-C and HAM-D-17 it was 0.93 (
30). The Cronbach’s α of QIDS-SR and QIDS-C have ranged from 0.86 to 0.87 in previous reports (
28,
30,
31). The QIDS-SR served as the primary measure of depressive symptoms. The QIDS-C was completed by the clinician to monitor symptom changes and guide treatment decisions.
Work Productivity and Impairment Questionnaire.
The Work Productivity and Impairment questionnaire, a six-item self-report scale, has good construct validity and test-retest reliability (
32). Items include employment status (item 1); number of hours missed from work in the last week due to health reasons (range: 0–80; item 2); number of hours missed from work due to other reasons such as vacation (item 3); number of hours worked in the last week (range: 0–80; item 4); impairment resulting from health conditions while working using a scale of 0–10, where 0 indicates no impairment (item 5); and impairment in regular daily activities other than work or job (item 6).
Using the scoring guide by Reilly (
33), absenteeism (percent of time missed from work), presenteeism (percent of impairment while working), and work productivity loss (percent of overall work impairment) were calculated using formulas based on items 2, 4, and 5. These scores are expressed as percentages, with higher scores reflecting greater impairment, and they comprised the primary work outcome measures for the present study.
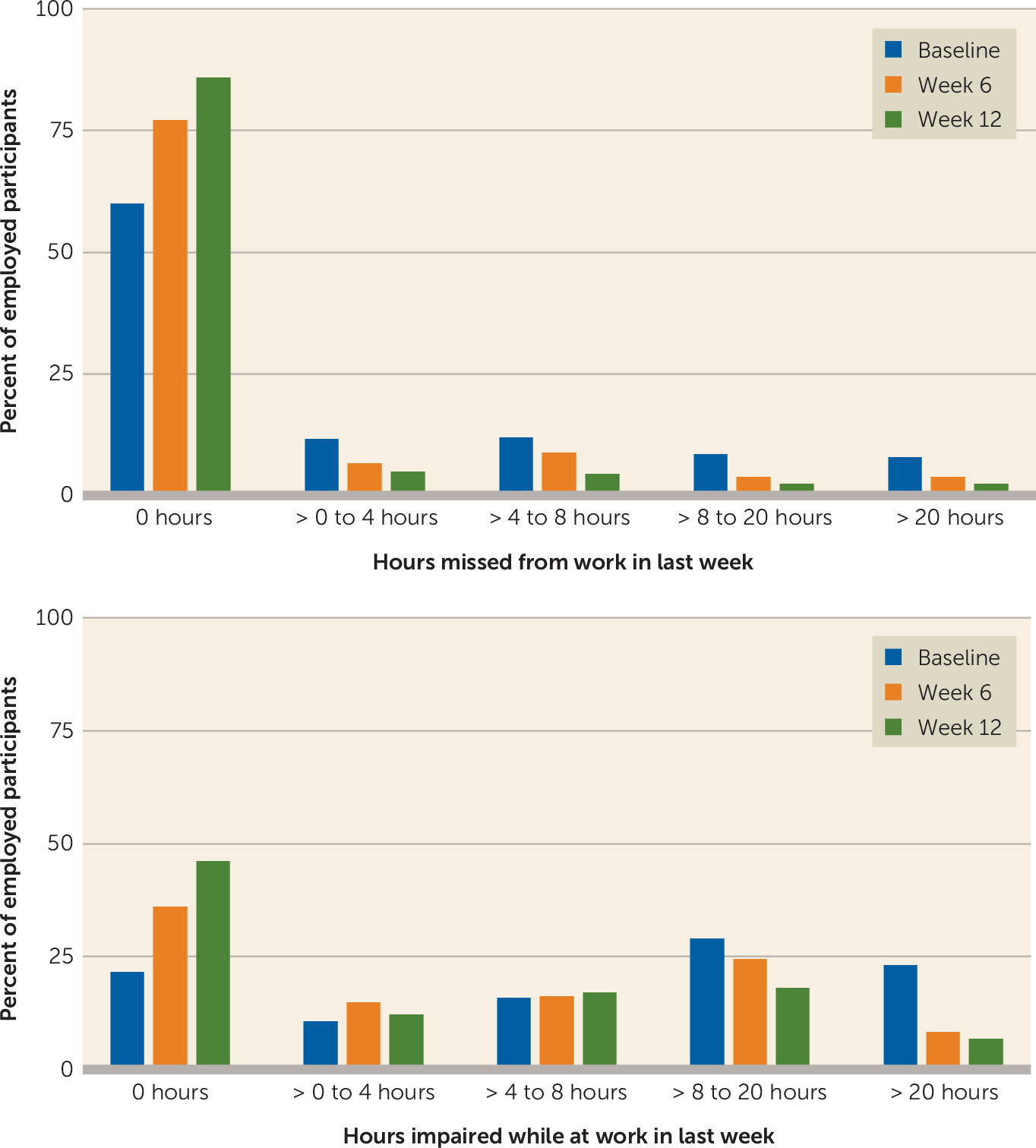
In addition to these scores, the number of hours lost to impaired productivity while at work was calculated using the following formula: (item 4)*(item 5/10). And this was reported along with item 2 in the following categories: none (0 hours), >0–4 hours (half a day of work), >4–8 hours (whole day of work), >8–20 hours (half a week of work), and >20 hours (greater than half a week of work).
The Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ).
The total score of this seven-item self-report questionnaire is calculated by adding the following items: motivation/interest, wakefulness/alertness, energy, ability to focus, ability to remember, ability to find words, and sharpness/mental acuity. The Pearson product-moment correlation coefficient between the CPFQ and HAM-D-17 ranged from 0.24 to 0.29 in a previous report (
34). The Cronbach’s α of the CPFQ has ranged from 0.89 to 0.91 in previous reports (
34,
35).
Clinical improvement.
Symptomatic remission at 12 weeks, the primary outcome of CO-MED, was ascribed if out of the last two consecutive QIDS-SR scores at least one was less than 6, while the other was less than 8. As described by Rush et al. (
26), this definition of remission in the CO-MED trial was chosen a priori “to ensure that a single ‘good week’ was not falsely signaling remission” in lieu of the conventional criteria of remission (QIDS-SR score ≤5) for 1 week (
36). Remission was measured at week 28 in a similar fashion.
Participation beyond 12 weeks (continuation phase) was contingent on whether participants received an acceptable benefit (defined as a QIDS-C score of 9 or less by week 12) or had reached a score of 10–13 on the QIDS-C and both the study physician and the participant decided to continue treatment because of substantial benefit.
Statistical Analyses
The analytic sample for this study included all CO-MED participants who were employed when they enrolled in the study (331 out of 665 [49.8%]). Based on the literature, we included the following baseline clinical and sociodemographic features to evaluate their role as predictors of change in work productivity outcomes: age, education (less than 12 years, 12–15 years, and ≥16 years of schooling), monthly income (<$2,000, $2,000–$4,000, and greater than $4,000), gender, race (white, black, other), Hispanic ethnicity, presence of anxious features at baseline, onset of depressive symptoms before age 18, presence of suicidal ideations at baseline, and baseline levels of depressive symptom severity, cognitive functioning, and each work productivity outcome (
5,
13–
15).
Because participation in the continuation phase of CO-MED was based on clinical improvement (
26), statistical analyses were conducted separately for the acute and continuation phases. It is noteworthy that because the treatment arms were not significantly different with respect to depression outcomes in the primary report (
26), we did not hypothesize a differential effect of treatment arm on work productivity outcomes; however, we did include treatment arm in the analysis to evaluate whether such an effect was present.
We estimated the change over time in each work productivity outcome (absenteeism, presenteeism, and work productivity loss) using repeated-measures analysis of variance with visit as the within-subject factor and all other variables as between-subject factors. We used linear mixed-model analyses with QIDS-SR and CPFQ scores at each visit, along with baseline covariates and baseline levels of each work productivity outcome and baseline CPFQ and QIDS-SR scores. For the continuation phase, we included scores on the QIDS-SR and CPFQ and each work productivity outcome at week 12 as additional covariates in the analyses. We estimated the correlation coefficients between work productivity outcome measures and depression severity, as well as the nine symptom domains included in the QIDS-SR. Because the variability of observations between participants is usually higher compared with the repeated observations in a single participant (
37), we calculated the correlation coefficients based on repeated observations over time using PROC MIXED as implemented in SAS (
38).
To develop groupings based on changes in work productivity during the first 6 weeks of acute-phase treatment, we used PROC TRAJ as implemented in SAS to estimate discrete mixture models with censored normal distribution (
39). For both linear and nonlinear (two-, three-, and four-degree polynomials) models, we increased the number of groupings in a stepwise fashion while evaluating changes in the model fit using Bayesian information criteria and Akaike information criteria with the lowest value indicating the best model fit (
40). We selected the final model based on fit and parsimony, group size, and interpretability. The groups were then compared with chi-square or general linear model for baseline clinical and sociodemographic features. Association of these groups with changes in depression severity after week 6 of CO-MED and remission at 3 months and 7 months were estimated using mixed models and logistic regression (first univariate and then multivariate analyses with backward elimination), respectively, after controlling for the above-mentioned baseline clinical and sociodemographic variables.
We used Bonferroni correction for multiple comparisons when indicated, set the level of significance at 0.05, and have reported all p values after adjustment for multiple comparisons. All analyses were done using SAS 9.3 (SAS, Inc., Cary, N.C.).
Results
Of the 665 CO-MED participants, 331 (49.8%) were employed at baseline and did not differ on baseline sociodemographic or clinical variables among the three treatment arms (
Table 1). During both the acute and continuation phases, work productivity loss and presenteeism were very highly correlated (0.94–0.95), while the correlation coefficients of absenteeism and presenteeism ranged from 0.37 to 0.47. Using conventional criteria proposed by Cohen (
41), there was a medium (>0.3) correlation between presenteeism and work productivity loss with the following depressive symptom domains: sad mood, concentration, general interest, energy level, and psychomotor agitation/retardation (also see
Table 2).
Work Productivity Improvement With Antidepressant Medications
Acute phase.
The percentage of participants who missed at least 1 hour of work in the last 7 days dropped from 40% at baseline to 14.1% at week 12. The percentage of participants who reported more than 20 hours of impairment while at work fell from 23.1% at baseline to 6.8% at week 12 (
Figure 1). All work productivity outcomes significantly improved after 12 weeks of treatment. The mean rate of absenteeism (percent of time missed from work) reduced significantly (F=6.34, df=7, 1606, p<0.0001), from 12.34 hours (SD=22.8) at baseline to 4.17 hours (SD=13.9) at 12 weeks; the mean rate of presenteeism (percent of impairment while at work) decreased significantly (F=22.82, df=7, 1707, p<0.0001), from 40.4 hours (SD=31.5) at baseline to 19.8 hours (SD=22.5) at 12 weeks; and the mean rate of work productivity loss (overall work impairment that includes absenteeism and/or presenteeism) lowered significantly (F=22.85, df=7, 1632, p<0.0001), from 44.9 hours (SD=32.4) at baseline to 21.0 hours (SD=27.4) at week 12. These reductions with time in absenteeism (F=4.34, df=6, 1227, p=0.0002), presenteeism (F=4.49, df=6, 1292, p=0.0002), and work productivity loss (F=5.79, df=6, 1213, p<0.0001) continued to be significant even after controlling for QIDS-SR and CPFQ scores at each visit, baseline clinical and sociodemographic variables along with baseline QIDS-SR and CPFQ scores and baseline absenteeism, presenteeism, and work productivity loss, respectively.
Continuation phase.
Changes during the continuation phase were not statistically significant after controlling for changes in QIDS-SR scores, CPFQ scores, baseline clinical and sociodemographic variables along with baseline and 12-week scores on the QIDS-SR and CPFQ and baseline and 12-week levels of each work productivity outcome (absenteeism [F=2.13, df=3, 427, p=0.10], presenteeism [F=1.31, df=3, 452, p=0.27], or work productivity loss [F=0.93, df=3, 413, p=0.43]).
During the acute phase, an educational level of college or higher was associated with lower absenteeism, presenteeism, and work productivity loss. Other baseline variables, excluding baseline levels of each work productivity outcome, did not significantly predict change in work productivity outcomes during either the acute or continuation phase (for detailed results, see Table S1 in the data supplement accompanying the online version of this article). Changes in CPFQ scores were significantly associated with work productivity changes during both the acute and continuation phases (see Table S1 in the online data supplement). Additionally, during both the acute and continuation phases, the bupropion and venlafaxine combinations did not differ from SSRI monotherapy for any of the work productivity outcomes.
Groups Based on Change in Work Productivity Within the First 6 Weeks of Treatment
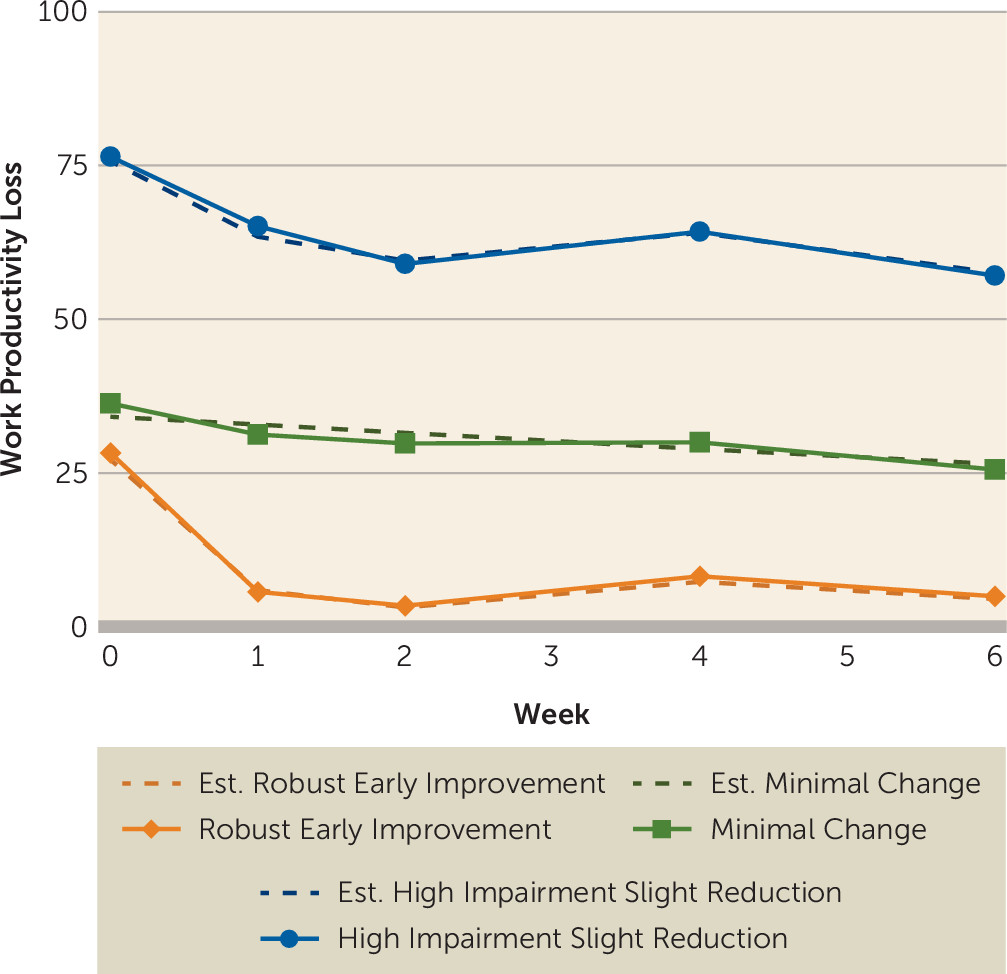
The final model (
Figure 2) identified the following three trajectories of change in work productivity during the first 6 weeks of the acute phase: 1) robust early improvement (23.8% of participants), 2) minimal change (49.2% of participants), and 3) high-impairment slight reduction (27.0% of participants). The model fit estimates for linear and nonlinear models with step-wise increase in the number of groupings, and maximum likelihood estimates of the final model parameters are presented in Tables S2 and S3, respectively, in the
online data supplement. At baseline, the three groups did not differ with regard to clinical and sociodemographic variables, except depression severity; the high-impairment slight reduction group had higher baseline QIDS-SR scores than participants in the robust early impairment and minimal change groups (see Table S4 in the
data supplement).
Association of Groups Defined by Early and Robust Change in Work Productivity With Subsequent Clinical Improvement
Participants with robust early improvement in work productivity had significantly higher unadjusted remission rates at 3 and 7 months compared with those with minimal change (3 months odds ratio=3.1, 95% confidence interval [CI]=1.5–6.6; 7 months odds ratio=2.9, 95% CI=1.2–6.8) and high-impairment slight reduction (3 months odds ratio=7.7, 95% CI=3.2–18.5; 7 months odds ratio=7.1, 95% CI=2.7–19.2). After controlling for remission status at week 6 and baseline clinical and sociodemographic variables, participants with robust early improvement continued to have significantly higher remission rates at both 3 months (odds ratio=5.4, 95% CI=1.8–15.9) and 7 months (odds ratio=5.0, 95% CI=1.5–16.4) when compared with those with high-impairment slight reduction. However, when compared with those with minimal change, those with robust early improvement had higher remission rates only at 3 months (odds ratio=2.7, 95% CI=1.0–6.9) but not at 7 months (odds ratio=2.2, 95% CI=0.8–6.1) (also see Table S5 in the online data supplement).
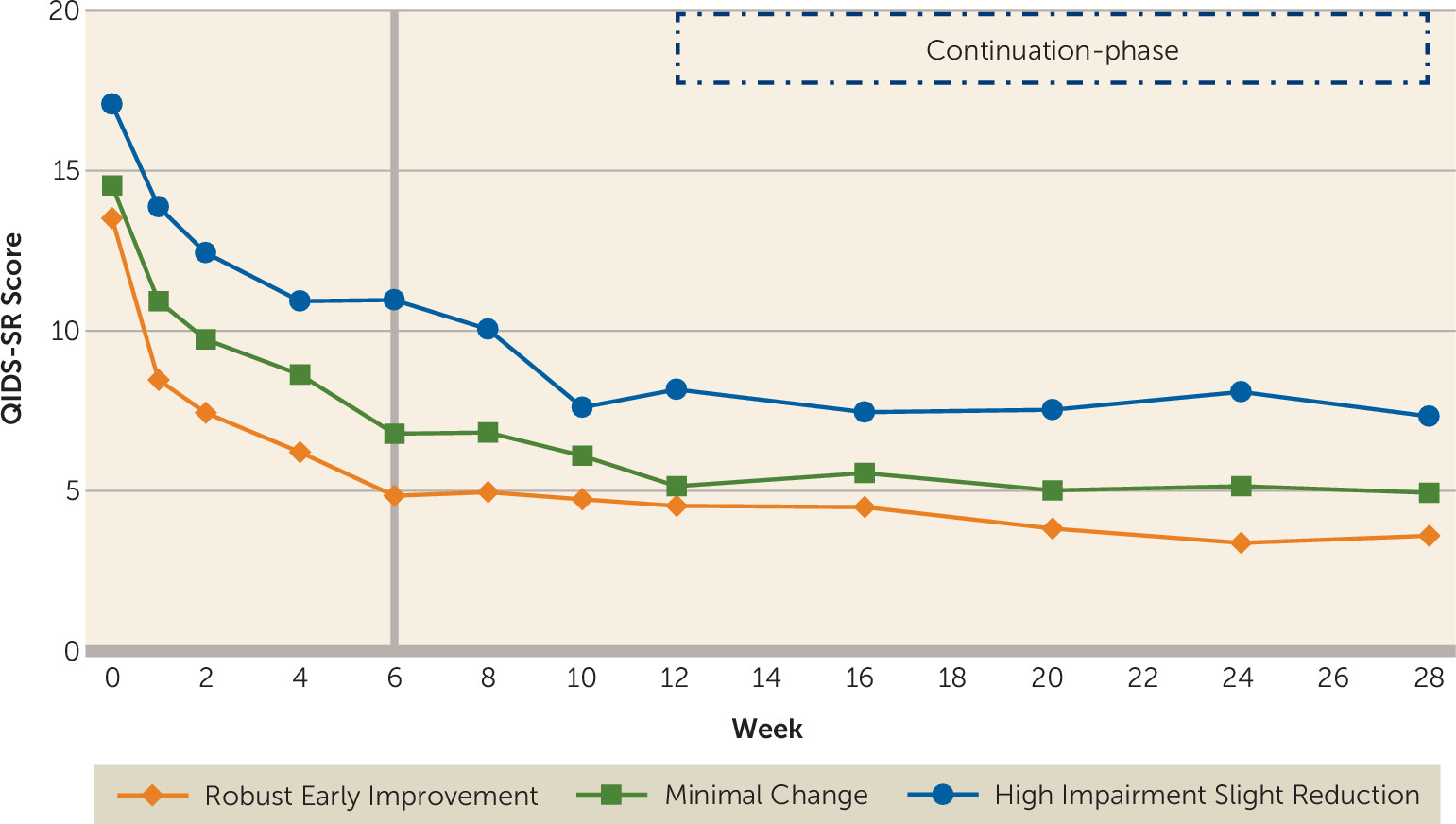
After controlling for baseline clinical and sociodemographic variables, participants in the three trajectory groups differed significantly (F=23.31, df=2, 258, p<0.0001) in depression severity from week 6 to week 12 of the acute phase, with a significant effect of time (F=9.34, df=3, 599, p<0.0001) and time-by-group interaction (F=3.20, df=6, 607, p=0.004) (
Figure 3). During the continuation phase and after controlling for the above-mentioned baseline variables, there was a significant difference in the levels of depression severity between the three groups (F=16.14, df=2, 231, p<0.0001), but depression severity did not change with time (F=1.31, df=4, 711, p=0.27) during this phase, nor was there a time-by-group interaction (F=1.19, df=8, 723, p=0.30) (also see
Figure 3).
Discussion
We found that work productivity improved with acute-phase antidepressant treatment even after accounting for change in depression severity and self-reported cognitive functioning. Secondly, the trajectories of this improvement in work productivity predicted long-term changes in depression severity and remission status. During the first 6 weeks of the trial, two groups of depressed participants (those with robust early improvement and those with minimal change) started at similar levels of work productivity and depression severity. One group attained an early and sustained reduction in work productivity loss, while the other experienced minimal change. The group with early improvement in work productivity had markedly lower levels of depression severity throughout the duration of the trial (28 weeks) and continued to be in remission at much higher rates at week 12, even after controlling for baseline clinical and sociodemographic variables and remission status at week 6. Early improvement in work productivity appears to be an important mediator of sustained remission.
These findings are consistent with prior reports that work productivity improves with antidepressant treatment (
5,
13–
15). In addition, the role of baseline levels of work productivity in predicting subsequent change with time (see Table S5 in the
online data supplement) is consistent with the findings of Beck et al. (
13). In line with the findings of Trivedi et al. (
5), we also found that baseline educational level moderated work productivity improvement with antidepressant treatment.
The high positive correlation between work productivity loss and presenteeism implies that a large portion of variance in overall work productivity loss is accounted for by presenteeism—that is, while employees are at their job, their productivity is substantially impaired. This result is consistent with epidemiological findings that presenteeism, compared with absenteeism, accounts for the majority of the work productivity impairment (
7,
8). Furthermore, our results that work productivity outcomes were similar for SSRI monotherapy and the bupropion and venlafaxine combinations are consistent with what was found with respect to depression symptom severity in the CO-MED trial.
Our findings that significant improvement in work productivity occurred even after accounting for baseline demographic and clinical factors, as well as changes in depression severity and self-reported cognitive functioning, support the importance of measuring work productivity during antidepressant treatment. Measures of depression severity fail to adequately capture the burden of depression (
42). Additionally, we found that changes in cognitive functioning were significantly associated with changes in work productivity. This report adds to the growing evidence regarding the importance of improving cognitive functioning in depressed patients. Our findings further support attainment of functional recovery as the ultimate outcome of antidepressant treatment (
43,
44).
The results of this study may have practical implication if our findings are replicated. Work productivity may be an easy-to-administer patient-centered outcome that can identify patients early in the course of treatment who may benefit with treatment change or augmentation. Among depressive symptom domains, energy and concentration are most strongly associated with work productivity outcomes. Hence, targeting these symptom domains with adjunct treatments may boost work productivity and improve long-term outcomes. Adjunctive occupational therapy has been shown to improve depression severity in the long-term (
45). Similarly, telephone-based coaching interventions focused on improving work productivity have been reported to improve depressive symptom severity in patients with dysthymia (
46). Perhaps such interventions could play a pivotal role in increasing the likelihood of sustained remission in patients with residual work impairment.
There are several limitations to our report. It is a secondary analysis. This study did not include any intervention that was meant to independently improve work productivity. Additionally, the work productivity measure in our study was subjective and based on self-report. Hence, it likely differs from objective measures of work productivity, such as those collected by employers. Furthermore, the generalizability of our findings to routine clinical care may be restricted by the high quality of treatment received by participants in the CO-MED trial. While adopted by the major treatment guidelines for decades, measurement-based care has not become routine practice.
In conclusion, we have demonstrated that work productivity outcomes improve significantly with antidepressant treatment and reflect burden of disease that is not captured adequately by depression severity alone. We have also demonstrated that early changes in work productivity are significant predictors of long-term clinical course. These findings highlight the multidimensional improvement with antidepressant treatment and argue for inclusion of work productivity assessments in routine clinical practice.
Acknowledgments
The authors thank the clinical staff at each clinical site for their assistance with this project; all of the study participants; and Eric Nestler, M.D., Ph.D., Carol A. Tamminga, M.D., and Savitha Kalidas, Ph.D., for administrative support.