Bipolar disorder, a mood disorder with an estimated prevalence of 2.4% (
1), is characterized by episodes of mania (pathologically elevated mood) and depression (pathologically lowered mood) interspersed with euthymic (normal) mood (
2,
3). Bipolar disorder has been ranked as the second leading cause of “days out of role” worldwide (
4), exacting high societal costs because of lost productivity (
5). Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder that affects 1%−5% of the population (
6,
7) and is characterized by inattention, hyperactivity, and impulsivity—symptoms that may partially overlap with those of bipolar disorder (
3,
8).
Bipolar disorder and ADHD commonly co-occur. Comorbid ADHD has been observed in up to 20% of people with bipolar disorder, and there is evidence that co-occurrence of these disorders is associated with a worse illness course than either disorder alone (
4,
9–
11). Research indicates that these disorders share common genetic etiologies (
11), but comorbid ADHD in bipolar disorder could still represent co-occurrence of two distinct pathophysiologies. Studies have, for example, suggested that patients with bipolar disorder who also have a history of childhood ADHD may represent a distinct early-onset subtype with specific neurobiological underpinnings (
9,
12).
In current practice guidelines, pharmacotherapy is a cornerstone of management for bipolar disorder and ADHD (
3). The foundation of pharmacological treatment of bipolar disorder often involves one or more medications classified as mood stabilizers, which can attenuate acute mood episodes and have a prophylactic effect against recurrent episodes and/or cycling. Although there is some debate about which medications should be classified as mood stabilizers, most experts agree that at least two medications—lithium salts and valproate—are indicated for both acute-phase therapy and prophylaxis of bipolar I disorder, and several second-generation antipsychotic drugs are increasingly used as mood stabilizers (
13). The therapeutics of ADHD differ considerably, and most patients will at some point be treated with central stimulants, which are presumed to increase neuronal signaling by marked elevation of the extracellular concentration of neurotransmitters in the prefrontal cortex of the brain (
14). The psychostimulant methylphenidate is one of the most widely used medications for ADHD in both the United States and the European Union, and its use in the treatment of adults with ADHD has increased in recent years (
15,
16).
There is a high rate of nonresponse and residual functional impairment in comorbid ADHD and bipolar disorder (
17), and the pharmacological treatment of comorbid ADHD in bipolar disorder presents a challenge. Not only may the treatment for bipolar disorder worsen ADHD symptoms (
18), clinicians have long worried that methylphenidate and other psychostimulants may also induce mania or even provoke psychosis (
19–
22). Some physicians even consider the use of psychostimulants to be contraindicated in bipolar disorder (
18). An analogous debate has centered on the use of antidepressants in bipolar disorder (for treatment of depressive episodes or co-occurring anxiety disorders) (
19–
23), although recent research indicates that this risk is reduced or eliminated by the use of a concomitant mood-stabilizing treatment (
21,
23).
A recent review of studies concerning the efficacy and safety of psychostimulants in adults with comorbid ADHD and bipolar disorder concluded that controlled studies and treatment guidelines were substantially lacking (
18). Only a handful of randomized controlled trials have studied stimulant use in comorbid ADHD and bipolar disorder (
24–
27), and all had small sample sizes and half of them focused on adolescents only (
26,
27). Nonetheless, these studies suggest that stimulants are effective in treating ADHD symptoms in bipolar disorder and produce no adverse mood elevation when used together with a mood stabilizer. In addition, open-label studies and case series suggest that the response to stimulants in comorbid ADHD and bipolar disorder appears to be similar to the response reported in adult ADHD (
18). Even though this is encouraging, the risk of treatment-emergent mania when psychostimulants are used in bipolar disorder needs to be further evaluated, both in larger studies and in real-life settings, before stimulant treatment can be recommended in bipolar disorder.
Large-scale observational data sets may be utilized to investigate combination therapies that are difficult or too costly to survey in a randomized controlled setting. But observational studies are hampered by confounding by indication—that is, subjects who receive a prescription for medication are inherently different from those who do not. However, several recent pharmacoepidemiological studies have employed within-individual study designs that better account for genetic and environmental confounders (
23,
28–
30). This design will automatically adjust for individual-specific confounders that normally restrict the usefulness of observational studies, including disorder liability, genetics, and stable environmental factors (e.g., family environment, early trauma, and so on).
The aim of this study was to examine the risk of treatment-emergent mania when methylphenidate is used in adults with bipolar disorder. We linked national Swedish registers and assessed the risk of manic events in the 3 months and the 3–6 months following initiation of methylphenidate therapy as compared with nontreated periods of equal length. We stratified the cohort by presence or absence of concomitant mood-stabilizing medication to explore whether mood-stabilizing medication influences the risk of treatment-emergent mania. By applying a within-individual design, we controlled for individual-specific confounders like mania liability, genetic makeup, and environmental factors.
Method
Subjects
Swedish national registries were linked with high accuracy using the unique Swedish national registration numbers assigned to all residents (
31). A search algorithm that has been demonstrated to yield a high positive predictive value was used to identify patients with bipolar disorder in the National Patient Register (
32), which includes all Swedish inpatient admissions since 1973 and all outpatient specialist admissions since 2001 (
33) and provides admission dates along with the main and eight secondary diagnosis codes in accordance with ICD classifications. Individuals were identified as having bipolar disorder if they had at least two inpatient or outpatient admissions with a discharge diagnosis of bipolar disorder (ICD-8 codes 296.00, 296.1, 296.3, 296.88, and 296.99; ICD-9 codes 296.0, 296.1, 296.3, 296.4, 296.8, and 296.9; ICD-10 codes F30 and F31). For the purposes of this study, we required that the diagnosis of bipolar disorder precede methylphenidate therapy. Additionally, to diminish the potential risk of misclassification, the included individuals were allowed a maximum of one inpatient or outpatient diagnosis of schizophrenia (ICD-8 code 295; ICD-9 code 295; ICD-10 code F25) and had to be 18 years of age at least 6 months before beginning methylphenidate treatment.
The study was approved by the regional ethics committee in Stockholm.
Exposure
Because methylphenidate is essentially the only psychostimulant used to treat comorbid ADHD in bipolar disorder in Sweden, it was the only one we studied. The period of exposure to methylphenidate was from July 2005 through 2013. Exposure status was ascertained using data from the Swedish Prescribed Drug Register, which provides data on all dispensed prescription drugs in Sweden (
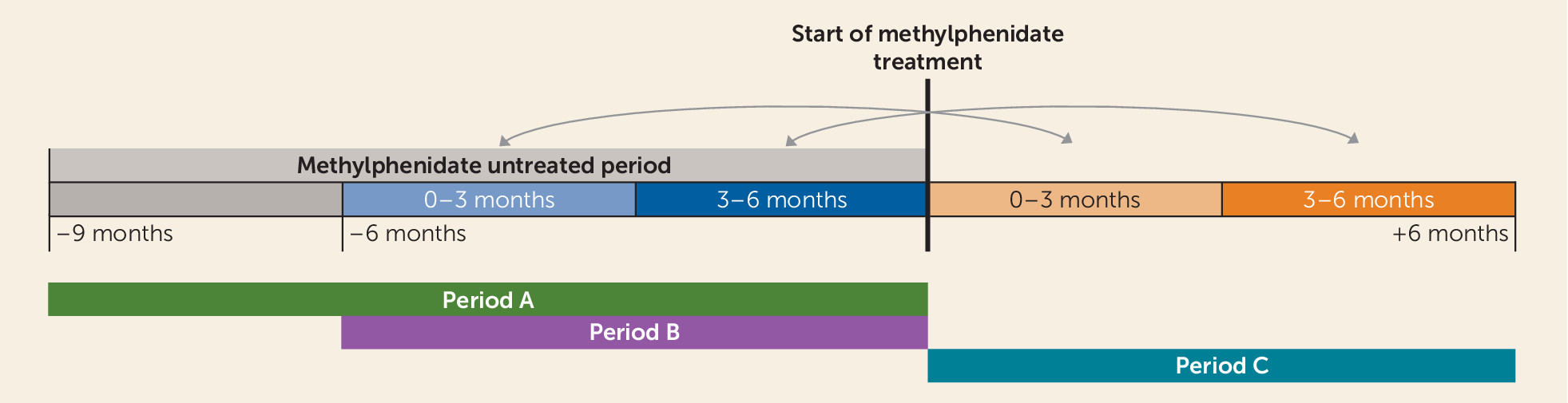
34). The register provides prescription and dispensation dates, medication name, dosage, package size, and a code in accordance with the Anatomical Therapeutic Chemical Classification System. Using this register, we identified dispensations of methylphenidate (N06BA04). To be included, an individual was required to have received a dispensation of methylphenidate without any other methylphenidate dispensations during the preceding 9 months (period A in
Figure 1), thereby ensuring that initiation of therapy occurred during that particular interval.
The cohort was next divided according to whether or not patients received dispensations of mood stabilizers—medications approved for either treatment of mania or prevention of recurrent bipolar episodes, including aripiprazole (N05AX12), lithium (N05AN01), olanzapine (N05AH03), quetiapine (N05AH04), and valproate (N03AG01). To be classified as being on continuous mood-stabilizing treatment, an individual had to have at least two dispensations of a mood stabilizer in the 9 months preceding the start of methylphenidate treatment (period A in
Figure 1), of which at least one had to occur within 6 months before the start of methylphenidate treatment (period B in
Figure 1). To be classified as not using a mood stabilizer, an individual could not have had any dispensations of aripiprazole, olanzapine, quetiapine, lamotrigine, lithium, valproate, carbamazepine, haloperidol, or risperidone during the 6 months preceding the start of methylphenidate treatment or at the date of the methylphenidate dispensation. Patients who were in neither of these groups were excluded.
Outcome
The occurrence of mania was ascertained using discharge diagnosis codes for mania (ICD-10 codes F30.0, F30.1, F30.2, F30.8, F30.9, F31.0, F30.1, and F30.2). A dispensation of an antipsychotic, lithium, or valproate after methylphenidate treatment could indicate treatment for a manic relapse or it could be a late safety measure. In analysis 1, dispensations of lithium, valproate, or antipsychotics (aripiprazole, olanzapine, quetiapine, haloperidol, or risperidone) to individuals without any dispensations of these drugs during the 9 months preceding methylphenidate medication (i.e., new dispensations) was interpreted as treatment for elevated mood and counted toward a treatment-emergent mania. Dosages of aripiprazole below 5 mg/day, olanzapine below 5 mg/day, and quetiapine below 100 mg/day were not considered. In a supplemental analysis, new dispensations of lithium and valproate were not counted toward elevated mood (see appendix 1 in the data supplement that accompanies the online edition of this article). In analysis 2, only a diagnosis of mania counted as treatment-emergent mania.
Statistical Analysis
In total, 65,683 individuals with bipolar disorder were identified in the Swedish National Patient Register, of whom 5,506 (8.4%) had at least one dispensation of methylphenidate between July 1, 2005, and December 31, 2013. Among these, 2,307 (41.9%) met the criteria of having a period of 9 months without receiving any other doses of methylphenidate prior to the medication initiation and being over 18 years of age.
Similar to a previous study of antidepressant-induced manic switch (
23), the effect of methylphenidate treatment on the rate of mania was estimated by Cox proportional hazards regression analyses using the STCOX command in STATA/IC, version 13. By conditioning on individual in the analyses (strata=individual), all subjects serve as their own control. This reduces confounding caused by differences between patients, including disorder severity (e.g., proneness to mania), genetic makeup, and environmental factors. We compared the rate of mania during a 6-month period before the start of methylphenidate treatment (period B in
Figure 1) with a 6-month period following the start of methylphenidate treatment (period C in
Figure 1). This provided the relative rate of mania following treatment, that is, the change or relative risk, rather than the absolute rate of mania, which may be inherently different between patients or patient groups (e.g., those with or without concomitant mood stabilizer treatment). To allow for assessment of both acute and more long-term effects (
20), interaction terms with split follow-up time (0–3 months, 3–6 months) were included in the statistical model. To account for deaths and migrations, we utilized the Cause of Death and Migration registries. If the patient died, emigrated, or was diagnosed with schizophrenia during the treatment period (period C in
Figure 1), the follow-up was censored at this time. In a supplemental analysis, a diagnosis of schizophrenia after methylphenidate treatment was counted toward mania (see appendix 2 in the
online data supplement).
Results
Table 1 presents descriptive statistics for the 2,307 subjects included in this study. Males made up 36.8% (N=848) of the cohort, and the distribution among the age groups 18–24 years, 25–39 years, and ≥40 years was 15.4%, 46.8%, and 37.8%, respectively. Among the 2,307 subjects in the study, 718 (31.1%) received no mood-stabilizing treatment, 1,103 (47.8%) met the criteria for being on mood-stabilizing treatment, and 486 (21.1%) did not meet either criterion and were not included in subsequent analyses. (For the types of stabilizing drugs dispensed, see appendix 3 in the
online data supplement.)
Methylphenidate and Risk of Mania Events Derived From Diagnoses and Dispensations of Stabilizing Medication
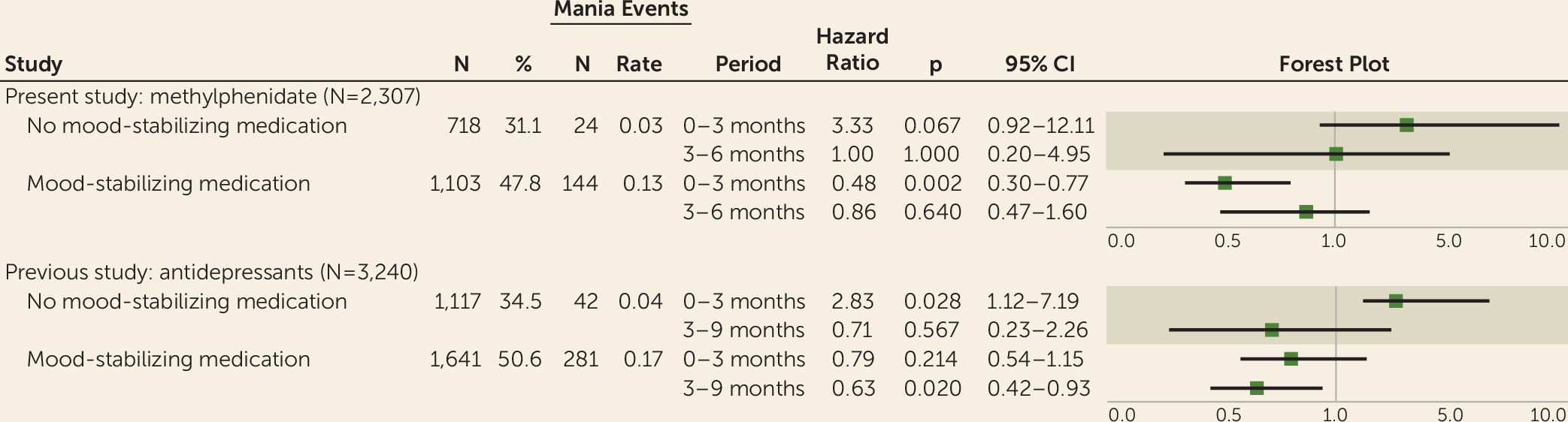
When we defined treatment-emergent mania as a diagnosis of mania or a new dispensation of an antipsychotic or a mood stabilizer, the absolute number of mania events during the complete 12 months of follow-up was lower among the 718 patients treated with methylphenidate monotherapy (N=61; mania rate=0.08) than among the 1,103 patients treated with a concurrent mood stabilizer (N=195; mania rate=0.18). However, the relative risk of mania following methylphenidate treatment among patients treated with methylphenidate monotherapy was increased in the first 3 months after treatment (hazard ratio=6.7, 95% CI=2.0–22.4) and in the subsequent 3 months (hazard ratio=9.7, 95% CI=2.9–31.7). By contrast, among patients treated with a concurrent mood stabilizer, the risk of mania was reduced in the initial 3 months (hazard ratio=0.6, 95% CI=0.4–0.9) and slightly reduced in the subsequent 3 months (hazard ratio=0.9, 95% CI=0.5–1.7) (
Table 2).
Methylphenidate and Risk of Mania Derived From Diagnoses Only
When treatment-emergent mania was defined using diagnoses of mania only, the absolute number of mania events was reduced but maintained a similar distribution. The relative risk of mania following methylphenidate medication in the monotherapy group was elevated, although not statistically significantly, in the initial 3 months after treatment (hazard ratio=3.3, 95% CI=0.9–12.1) and unchanged in the subsequent 3 months (hazard ratio=1.0, 95% CI=0.2–5.0). Similar to the first analysis, among patients treated with a concurrent mood stabilizer, the risk of mania was reduced in the initial 3 months (hazard ratio=0.5, 95% CI=0.3–0.8) and slightly reduced in the subsequent 3 months, although not statistically significantly (hazard ratio=0.9, 95% CI=0.5–1.6) (
Table 3).
Treatment-Associated Mania: Methylphenidate and Antidepressants
In a previous study (
23), we used similar data and methodologies to assess the risk of treatment-emergent mania due to antidepressant therapy in bipolar disorder.
Figure 2 contrasts the results from that study with those from the present study and suggests that concomitant stabilizing treatment attenuates the risk of medication-induced mania by methylphenidate and antidepressants in a similar fashion. In supplemental analyses, we found no evidence for an association or interaction between concomitant antidepressant treatment and mania after methylphenidate treatment (see appendix 4 on the
online data supplement).
Discussion
In the largest study to date (N=2,307), we examined the risk of treatment-emergent mania in patients with bipolar disorder when methylphenidate is taken alone or in combination with mood-stabilizing medication. We found that methylphenidate may be associated with treatment-emergent mania in patients on methylphenidate monotherapy, but no evidence was found for a positive association between methylphenidate and treatment-emergent mania among bipolar patients who were concomitantly receiving a mood stabilizer.
Previous research on the effectiveness and tolerability of stimulant medication in patients with bipolar disorder and comorbid ADHD is limited, both in number of studies and number of participants (
18,
24–
27). Furthermore, these studies are restricted to combinations of stabilizing medications and stimulants, and they survey different types of stimulants and different populations (adults or children). Nevertheless, our finding that methylphenidate in combination with a stabilizing medication is not associated with a risk of treatment-emergent mania is in line with previous studies. For example, an open-label study of 14 adult patients with bipolar disorder on stabilizing medication reported no incidences of adverse elevated mood after initiation of methylphenidate treatment (
25). Similarly, a 4-week placebo-controlled trial of methylphenidate in combination with mood stabilizers in 16 patients 5–17 years of age with bipolar disorder and comorbid ADHD found methylphenidate to be well tolerated and reported improved ADHD symptoms at the end of the study (
26). In another open-label study, 40 adult patients with comorbid ADHD and bipolar disorder were studied during 4 weeks of combination treatment with a mood stabilizer and the stimulant lisdexamfetamine (
24). The study found improved ADHD and depressive symptoms and no increased risk of treatment-emergent mania, which echoes findings in previous studies of methylphenidate (
25,
26). Finally, in a 4-week randomized controlled trial of 40 patients 6–17 years of age with comorbid ADHD and bipolar disorder, treatment with divalproex and amphetamine was found to be more effective in treating ADHD symptoms than divalproex and placebo (
27), with only one participant in the stimulant group experiencing mania.
Although there have been no randomized studies of methylphenidate monotherapy in bipolar disorder to which our findings can be contrasted, an earlier study (
23) of the risk of treatment-emergent mania due to antidepressant monotherapy in bipolar disorder, using a similar methodology, obtained results analogous to those of the present study (
Figure 2).
Given that up to 20% of patients with bipolar disorder may suffer from comorbid ADHD (
4,
9,
35), it is not surprising that 8.4% of all identified Swedish bipolar disorder patients received at least one prescription for methylphenidate during an 8-year period. Stabilizing medications effectively prevent mood episodes but may worsen attention and concentration, which can impair everyday functioning in this subgroup of patients. Although previous studies suggest that stimulant use in comorbid ADHD and bipolar disorder improves ADHD symptoms (
24–
27), a remaining concern has been whether stimulants are safe in bipolar disorder, particularly in terms of treatment-emergent mania. Our large-scale study indicating no increased risk of treatment-emergent mania when methylphenidate is used in combination with a mood stabilizer is thus reassuring, and it suggests that this combination may provide a welcome treatment option for this complicated patient group (
4,
9).
Strengths of this study include the large number of subjects and the naturalistic setting. The study includes individuals who, for various reasons, would have been excluded from or would not have volunteered for a clinical trial. Moreover, the use of a within-individual design automatically adjusts for a large number of individual-specific factors, including prior disorder severity, genetic makeup, and environmental factors, which would not be possible to adjust for in a conventional epidemiological approach. This provides an improved strategy for handling selection effects and confounding by indication in pharmacoepidemiological studies, which is exemplified by the difference in overall mania rates between the groups on monotherapy and on concomitant mood stabilizers.
A limitation of this study is that although the design allows for extensive adjustment of otherwise unmeasured confounders, it does not adjust for changes that might have occurred during the 12-month follow-up. Given the observational nature of the study, we cannot rule out the possibility that unmeasured factors may be specific for each group. Hence, the increased risk of mania in the monotherapy group may be attributable to untreated bipolarity rather than the methylphenidate treatment per se. It is thus important to confirm the findings in other samples and with other study designs.
It should be noted that the elevated risk of treatment-emergent mania in the monotherapy group was not statistically significant when only mania diagnoses were taken into account (p=0.067). Even though this is likely to be due to the small sample size of the monotherapy group, it highlights the fact that the number needed to harm is high (i.e., the risk of treatment-emergent mania is low). Nonetheless, patients in the monotherapy group likely lacked a mood-stabilizing treatment precisely because they had a history of lower rate of mania than those who were on a mood stabilizer (see the mania rates in
Table 2 and
Table 3). This is important to consider because if the patients treated with a mood stabilizer (with a higher underlying risk of mania) were put on methylphenidate monotherapy, they might present a lower number needed to harm.
Whereas the diagnosis of bipolar disorder in the Swedish National Patient Register has been validated (
32), the diagnosis of acute mania has not. The diagnosis of acute mania in a patient with known bipolar disorder is, however, likely to be valid. It should be noted, however, that even though this study is likely to have accurately captured the severe end of the mania spectrum, it may have failed to capture hypomania and elated mood that did not lead to a hospitalization. This is why we combined diagnoses of mania and new dispensations of antipsychotic medication at dosages that correspond with an antimanic treatment. In doing so, 37 additional mania events (5.2%) were observed in the monotherapy group (N=718), whereas 51 additional mania events (4.6%) were observed in the mood stabilizer group (N=1,103). It is further possible that changes in type or dosage of stabilizing medication also indicate elevated mood. However, this was not considered, given the ambiguity of such changes. It should also be noted that new adjunctive stabilizing treatment might not represent a manic relapse but rather a late safety measure by a treating clinician. Furthermore, the diagnoses in the National Patient Register can only identify bipolar disorder as a whole—they cannot distinguish between bipolar I and bipolar II disorder. It is possible that patients with these two different presentations of bipolar disorder respond differently to methylphenidate treatment, which warrants further research.
Our study focused only on treatment-emergent mania. We did not assess the effectiveness of the stimulant medication on ADHD symptoms. The study can thus neither inform us about the risk-benefit ratio of using stimulants in bipolar disorder nor elucidate potential synergistic effects of the combination of methylphenidate and mood stabilizer treatment. Lastly, because of the large number of different drugs used in the treatment of bipolar disorder, specific stabilizing medications could not be studied individually.
Conclusions
This study suggests that methylphenidate may increase the risk of treatment-emergent mania in patients suffering from bipolar disorder when it is used without a concomitant mood-stabilizing treatment. On the basis of this finding, we recommend careful assessment to rule out bipolar disorder before initiating methylphenidate as a monotherapy. As no association with treatment-emergent mania was observed among bipolar patients who were concomitantly receiving a mood-stabilizing medication, it would appear that concomitant therapy of ADHD is both safe and feasible in the context of ongoing preventive therapy. Although this study utilized a within-individual design to better handle confounding, the study used observational data, so not all potential confounding can be ruled out. Therefore, more research is warranted in this important area to further elucidate the potential mania-inducing properties of methylphenidate and the extent to which stabilizing drugs hamper this adverse reaction.