Trajectories of Response to Dorsolateral Prefrontal rTMS in Major Depression: A THREE-D Study
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Participants
Study Design
rTMS Procedure
Measures
Clinical assessments and prognostic factors.
Outcomes.
Statistical Analysis
Results
Response Trajectories
| Number of Groups | BIC | 2×ΔBICa |
|---|---|---|
| 1 | –5918.38 | NA |
| 2 | –5640.88 | 555 |
| 3 | –5522.37 | 237.02 |
| 4 | –5496.22 | 52.3 |
| 5 | –5552.18 | –111.92 |
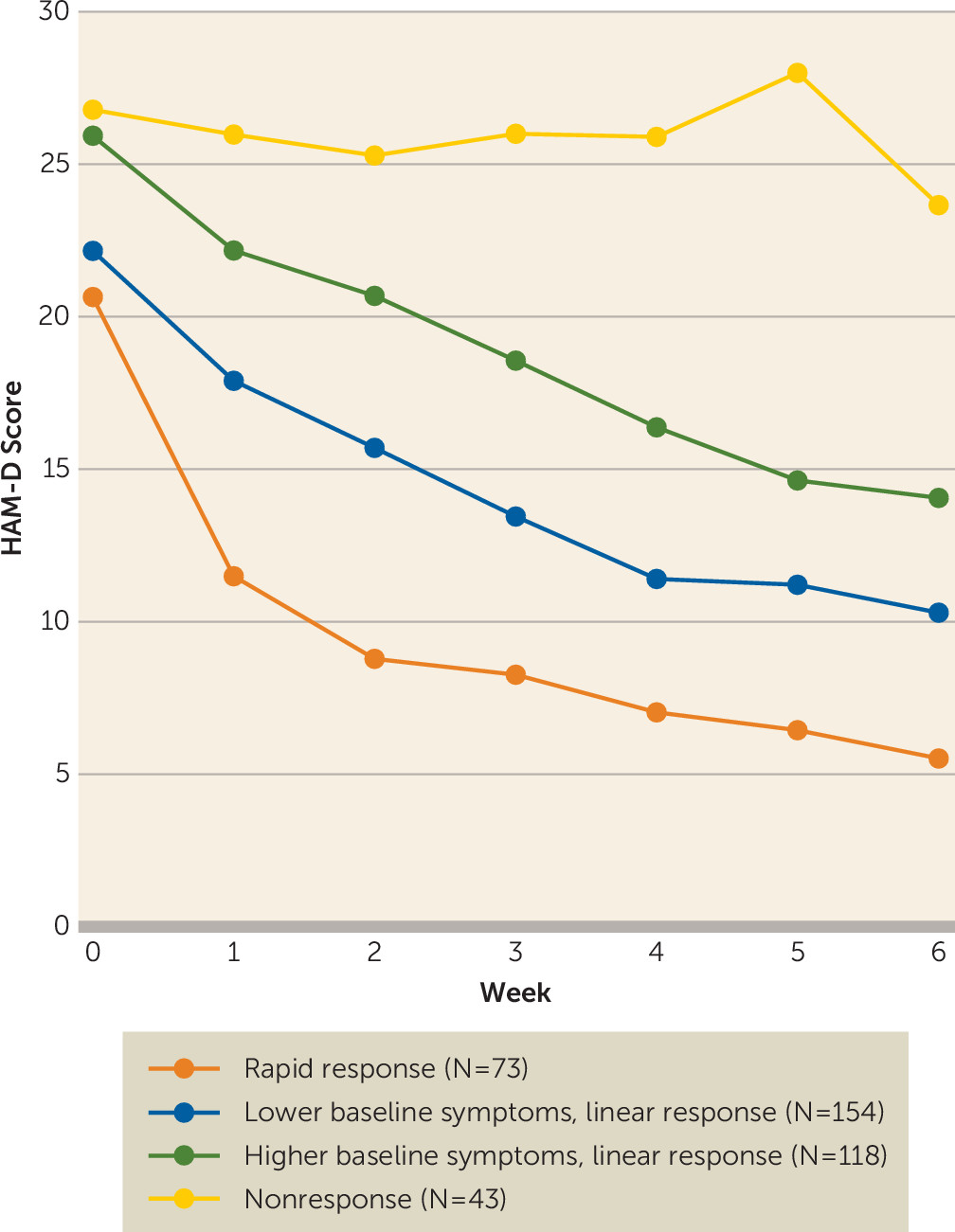
| Variable | Total Sample (N=388) | Rapid Response (N=73) | Lower Baseline Symptoms, Linear Response (N=154) | Higher Baseline Symptoms, Linear Response (N=118) | Nonresponse (N=43) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 42.3 | 11.5 | 45.4 | 11.1 | 41.2 | 11.4 | 42.9 | 11.3 | 39.5 | 12.1 |
| Education (years) | 16.3 | 3.1 | 16.7 | 2.8 | 16.0 | 2.8 | 16.7 | 3.3 | 15.8 | 3.7 |
| Age at depressive symptom onset (years) | 20.9 | 10.9 | 21.9 | 12.2 | 19.9 | 9.0 | 22.0 | 11.9 | 19.6 | 11.9 |
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Duration of current episode (months) | 14.0 | 8.0, 25.0 | 18.0 | 11.0, 30.0 | 12.0 | 6.0, 24.0 | 17.0 | 10.0, 28.0 | 14.0 | 7.0, 24.0 |
| N | % | N | % | N | % | N | % | N | % | |
| Male | 159 | 41.0 | 32 | 43.8 | 67 | 43.5 | 41 | 34.7 | 19 | 44.2 |
| Unemployed | 243 | 62.6 | 34 | 46.6 | 96 | 62.3 | 83 | 70.3 | 30 | 69.8 |
| Right-handed | 345 | 88.9 | 60 | 82.2 | 139 | 90.3 | 108 | 91.5 | 38 | 88.4 |
| Treatment measures | ||||||||||
| Antidepressant treatment | 295 | 76.0 | 59 | 80.8 | 121 | 78.6 | 79 | 66.9 | 36 | 83.7 |
| Antidepressant augmentation | 71 | 18.3 | 12 | 16.4 | 31 | 20.1 | 20 | 16.9 | 8 | 18.6 |
| Antidepressant combination | 84 | 21.6 | 19 | 26.0 | 32 | 20.8 | 27 | 22.9 | 6 | 14.0 |
| Benzodiazepine use | 123 | 31.7 | 14 | 19.2 | 52 | 33.8 | 36 | 30.5 | 21 | 48.8 |
| Psychotherapy | 151 | 38.9 | 21 | 28.8 | 70 | 45.5 | 44 | 37.3 | 16 | 37.2 |
| Number of adequate antidepressant trials | ||||||||||
| None | 30 | 7.7 | 3 | 4.1 | 10 | 6.5 | 15 | 12.7 | 2 | 4.7 |
| One | 173 | 44.6 | 34 | 46.6 | 70 | 45.5 | 50 | 42.4 | 19 | 44.2 |
| Two | 111 | 28.6 | 28 | 38.4 | 46 | 29.9 | 26 | 22.0 | 11 | 25.6 |
| Three | 74 | 19.1 | 8 | 11.0 | 28 | 18.2 | 27 | 22.9 | 11 | 25.6 |
| History of ECT treatment | 18 | 4.6 | 0 | 0.0 | 4 | 2.6 | 8 | 6.8 | 6 | 14.0 |
| Any anxiety comorbidity | 207 | 53.4 | 28 | 38.4 | 86 | 55.8 | 69 | 58.5 | 24 | 55.8 |
| Baseline symptom scales | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| HAM-D | 23.5 | 4.3 | 20.6 | 2.7 | 22.2 | 3.5 | 25.9 | 4.0 | 26.8 | 4.3 |
| QIDS-SR | 17.0 | 3.9 | 13.8 | 3.3 | 16.8 | 3.7 | 18.1 | 3.3 | 19.8 | 3.3 |
| Brief Symptom Inventory | 10.0 | 5.2 | 7.5 | 5.0 | 9.3 | 4.8 | 11.3 | 5.4 | 13.0 | 4.6 |
| Treatment characteristics | ||||||||||
| N | % | N | % | N | % | N | % | N | % | |
| HFL rTMS | 189 | 48.7 | 35 | 47.9 | 81 | 52.6 | 56 | 47.5 | 17 | 39.5 |
| iTBS rTMS | 199 | 51.3 | 38 | 52.1 | 73 | 47.4 | 62 | 52.5 | 26 | 60.5 |
| Total Sample (N=388) | Rapid Response (N=73) | Lower Baseline Symptoms, Linear Response (N=154) | Higher Baseline Symptoms, Linear Response (N=118) | Nonresponse (N=43) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | N | % | N | % | N | % | N | % | N | % | χ2 | p |
| Response | ||||||||||||
| Week 1 | 35 | 9.0 | 28 | 38.4 | 5 | 3.3 | 2 | 1.7 | 0 | 0.0 | 94.78 | <0.0001 |
| Week 2 | 75 | 19.3 | 52 | 71.2 | 20 | 13.0 | 3 | 2.5 | 0 | 0.0 | 161.72 | <0.0001 |
| Week 3 | 109 | 28.1 | 51 | 69.9 | 47 | 30.5 | 11 | 9.3 | 0 | 0.0 | 100.88 | <0.0001 |
| Week 4 | 179 | 46.1 | 63 | 86.3 | 85 | 55.2 | 30 | 25.4 | 1 | 2.3 | 106.06 | <0.0001 |
| Final | 181 | 46.7 | 64 | 87.7 | 83 | 53.9 | 34 | 28.8 | 0 | 0.0 | 105.29 | <0.0001 |
| Remission | ||||||||||||
| Week 1b | 9 | 2.3 | 9 | 12.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Week 2b | 25 | 6.4 | 22 | 5.7 | 3 | 2.0 | 0 | 0.0 | 0 | 0.0 | ||
| Week 3 | 33 | 8.5 | 28 | 38.4 | 5 | 3.3 | 0 | 0.0 | 0 | 0.0 | 104.03 | <0.0001 |
| Week 4 | 50 | 12.9 | 38 | 52.0 | 10 | 6.5 | 2 | 1.7 | 0 | 0.0 | 124.90 | <0.0001 |
| Final | 111 | 28.6 | 58 | 79.4 | 43 | 27.9 | 10 | 8.5 | 0 | 0.0 | 133.08 | <0.0001 |
Characteristics Associated With Each Symptom Trajectory
| Rapid Response (N=73) | Lower Baseline Symptoms, Linear Response (N=154) | Higher Baseline Symptoms, Linear Response (N=118) | Nonresponse (N=43) | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Selected covariates | ||||||||
| Age | 1.04 | 1.01–1.07 | 1.00 | (Reference) | 1.00 | 0.98–1.03 | 0.98 | 0.94–1.01 |
| Benzodiazepine useb | 0.40 | 0.18–0.90 | 1.00 | (Reference) | 0.88 | 0.47–1.64 | 2.25 | 0.99–5.11 |
| Baseline HAM-D | 0.90 | 0.80–1.01 | 1.00 | (Reference) | 1.30 | 1.19–1.42 | 1.31 | 1.17–1.47 |
| Baseline QIDS-SR | 0.79 | 0.71–0.87 | 1.00 | (Reference) | 1.02 | 0.93–1.11 | 1.20 | 1.05–1.38 |
| A priori covariate | 1.00 | (Reference) | ||||||
| iTBS rTMSc | 1.02 | 0.53–1.98 | 1.00 | (Reference) | 1.27 | 0.71–2.29 | 1.87 | 0.82–4.28 |
Discussion
Limitations
Conclusions
Acknowledgments
Footnotes
Supplementary Material
- View/Download
- 805.08 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

