Lithium Exposure During Pregnancy and the Postpartum Period: A Systematic Review and Meta-Analysis of Safety and Efficacy Outcomes
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Search Strategy
Eligibility Criteria
Meta-Analysis Primary and Secondary Outcomes, and Qualitative Synthesis
Data Extraction
Evidence Synthesis
Results
Synthesis of the Search Results and Main Characteristics of the Included Studies
| First Author, Year (Reference) | Sample Size (N) | Source | Lithium Exposure | Comparison | Main Safety or Tolerability Results (Systematic Qualitative Review) |
|---|---|---|---|---|---|
| Safety: case-control studies | |||||
| Edmonds 1990 (38) | 68 | Birth Defects Monitoring Program (USA) | Pregnancy | Lithium exposure in births with versus without Ebstein’s anomaly | Among the 34 infants with confirmed Ebstein’s anomaly and the infants in the control group, we found none whose mother had a history of manic-depressive illnesses or lithium use during pregnancy. |
| Zalzstein 1990 (39) | 227 | Department of Pediatrics and the Research Institute, Hospital for Sick Children, Toronto | Pregnancy (first trimester) | Lithium exposure in births with Ebstein’s anomaly versus lithium exposure in births with neuroblastoma | One case of in utero lithium exposure in the 168 mother-child-pair control group with neuroblastoma, but no cases in mothers of 59 children with Ebstein’s anomaly. The results can rule out (with 80% power and an alpha of 0.05) increased risk of more than 28-fold. This potential risk is much lower than the historically claimed 500-fold computed from the uncontrolled Danish registry (40). |
| Czeizel 1990 (41) | 32,224 | Hungarian Case-Control Surveillance of Congenital Anomalies | Pregnancy | Lithium exposure in births with versus without cardiac anomaly | No statistically significant association was found between the use of lithium and the appearance of any congenital anomaly. |
| Boyle 2017 (42) | 114,832 | 15 congenital anomaly registries in the European Surveillance of Congenital Anomalies | Pregnancy (first trimester) | Lithium exposure in births with Ebstein’s anomaly versus lithium exposure in births with another cardiac anomaly | Ebstein’s anomaly was associated with maternal mental health problems generally rather than lithium or benzodiazepines specifically; therefore, changing or stopping medications may not be preventive. |
| Lisi 2010 (43) | 18,131 | International Clearinghouse of Birth Defects Surveillance and Research | Pregnancy (first trimester) | Lithium exposure in patients with versus without malformation | No statistically significant association was found between malformations and the use of lithium. |
| Safety: prospective cohort studies | |||||
| Schou 1976 (44) | 120 | Lithium Baby Register (Scandinavia) | Pregnancy (first trimester) | Physical and mental development of non-malformed babies exposed to lithium versus their unexposed siblings | This was is a questionnaire follow-up of the physical and mental development of lithium children who were not malformed at birth. Sixty lithium-exposed children were examined, and their unexposed siblings served as a control group. The data do not reveal any increased frequency of physical or mental anomalies among the lithium-exposed children. |
| Jacobson 1992 (26); included in the meta-analysis | 286 | Four teratogen information centers in the United States and Canada | Pregnancy (first trimester) | Malformations and perinatal complications in lithium exposure versus no exposure | No statistically significant difference was found between groups in total malformation rate (2.8% lithium group, 2.4% control group), and no difference was found in risk ratio for congenital malformation, cardiac malformation, or Ebstein’s anomaly. Similar rates of spontaneous abortion were seen in the lithium and nonlithium groups (9% and 8%), as well as similar rates of premature delivery (4% and 5%). |
| Diav-Citrin 2014 (2); included in the meta-analysis | 1,003 | Israeli Teratology Information Service (Jerusalem) | Pregnancy (first trimester) | Malformations and perinatal complications in lithium exposure versus no exposure, and versus controls | There were significantly more miscarriages and elective terminations in pregnancies exposed to lithium (16.4% and 9.3%) and among mothers with bipolar disorder (8.3% and 8.3%) than in an unexposed group (5.7% and 2%). The rate of preterm deliveries was significantly higher in the lithium group (13.7%) and the bipolar disorder group unexposed to lithium (10.2%) compared with unexposed pregnancies (6.0%). The rate of major congenital anomalies after exclusion of genetic or cytogenetic anomalies was not significantly different among the three groups from the Israeli register. However, when data from Australia and Canada were also considered, babies exposed to lithium had significantly higher rates of major anomalies without chromosomal or genetic conditions (8.6% and 2.5%), cardiovascular anomalies (3.9% and 0.5%), cardiovascular anomalies excluding resolved cases (2.6% and 0.2%), and non-cardiovascular anomalies (5.9% and 2%). |
| Newport 2005 (34) | 24 | Women’s Mental Health Program Delivery Information Sheet (USA) | Pregnancy and postpartum | Perinatal complications in high-lithium versus low-lithium exposure | The rate of all complications except gestational diabetes was consistently higher in the high lithium exposure group. In particular, the rates of CNS and neuromuscular complications were significantly higher, the duration of infant hospital stays was significantly longer, and 1-minute Apgar scores were significantly lower in the high lithium exposure group. The rates of preterm delivery, low birth weight, and infant respiratory complications were higher in the high lithium exposure group than in the low lithium exposure group, but the differences only approached significance. |
| Safety: retrospective cohort studies | |||||
| Schou 1973 (45) | 118 | Register of Lithium Babies (Scandinavia) | Pregnancy (first trimester) | Malformations in babies exposed to lithium | Of the 118 children exposed to lithium, five were stillborn and seven died within the first week of life; six of these twelve children had malformations. The total number of children with malformations was nine, of which two had Down’s syndrome. |
| Weinstein 1975 (46) | 143 | Register of Lithium Babies (USA) | Pregnancy | Malformations in babies exposed to lithium | The 143 cases of lithium use during pregnancy recorded by the register showed that infants exposed to lithium appeared to have a higher than expected ratio of cardiovascular anomalies (7.7%) to all anomalies (9.1%) and may have an increased risk of congenital heart disease. The author believes that these findings justify a conservative policy on the use of lithium with fertile and pregnant women. |
| Weinstein 1976 (47) | 166 | Register of Lithium Babies (USA) | Pregnancy | Malformations in babies exposed to lithium | The ratio of Ebstein’s anomaly to all reported nontrivial anomalies was 1:4.5; the ratio of Ebstein’s anomaly to all forms of congenital heart disease to all nontrivial malformations is 1:1.5. In the register, the ratio of malformations of the tricuspid valve and tricuspid atresia to all cardiac anomalies was about 1:2.4, and the ratio of tricuspid atresia to all congenital heart defects in the baseline studies was about 1:44. The maximum frequencies of congenital malformations reported to the register (10.8%) did not substantially exceed the expected incidence of such malformations in the general population. |
| Källén 1983 (25); included in the meta-analysis | 287 | Registry of congenital malformations, medical birth registry, discharge registry for inpatient psychiatric wards (Sweden) | Pregnancy (first trimester) | Malformations and perinatal outcomes babies exposed versus unexposed to lithium | There was no statistically significant difference between delivery outcome or malformations in women on lithium and women on other psychotropic drugs. None of the infants with heart disease had Ebstein’s anomaly. |
| Van der Lugt 2012 (48) | 30 | Perinatal Center, Leiden University Medical Center (Netherlands) | Pregnancy | Cognition at follow-up (3–15 years) in babies exposed to lithium | This study reports the long-term outcome of 30 children who were exposed to lithium in utero and were breastfed. One child had signs of a minor neurological dysfunction but without further clinical implications. The results of the cognitive tests were within normal limits. Growth, behavior, and general development were within the normal range. Neurological screening and growth measurements did not show any significant abnormalities in the children; all were well within the normal range. |
| Forsberg 2018 (27); included in the meta-analysis | 39 | Karolinska University Hospital, Stockholm | Pregnancy | Cognition at 5 years, and perinatal complications in lithium exposure versus no exposure, and in mood disorder versus controls | The children’s full-scale IQ, performance IQ, and verbal IQ results did not differ significantly between lithium-exposed and unexposed groups. The processing speed quotient was significantly lower in children exposed to mood disorders than control subjects. Similar rates of premature delivery and neonatal care and similar Apgar scores at 5 minutes are described across groups. |
| Patorno 2017 (11); included in the meta-analysis | 1,325,563 | U.S. Medicaid Analytic eXtract | Pregnancy (first trimester) | Cardiac malformations in babies exposed versus unexposed to lithium, and versus lamotrigine exposure | Cardiac malformations were present in 16 of the 663 infants exposed to lithium (2.41%), 15,251 of the 1,322,955 unexposed infants (1.15%), and 27 of the 1,945 infants exposed to lamotrigine (1.39%). The adjusted risk ratio for cardiac malformations among infants exposed to lithium compared with unexposed infants was 1.65 (95% CI=1.02–2.68). The risk ratio was 1.11 (95% CI=0.46–2.64) for a dosage ≤600 mg/day, 1.60 (95% CI=0.67–3.80) for 601–900 mg/day, and 3.22 (95% CI=1.47–7.02) for more >900 mg/day. The prevalence of right ventricular outflow tract obstruction defects was 0.60% among lithium-exposed infants and 0.18% among unexposed infants (adjusted risk ratio=2.66; 95% CI=1.00–7.06). Results were similar when lamotrigine-exposed infants were used as the reference group. |
| Frayne 2018 (28); included in the meta-analysis | 33 | Childbirth and Mental Illness Clinic (Australia) | Pregnancy | Malformations and perinatal complications in babies exposed versus unexposed to lithium | In the cohort of women exposed to lithium during the first trimester of pregnancy, there were no recorded congenital abnormalities Women with lithium prescriptions, irrespective of whether they continued or discontinued the medication, represented a high-risk group obstetrically and in antenatal complications (88%). |
| Troyer 1993 (29); included in the meta-analysis | 350 | International Register of Lithium Babies (Scandinavia) | Pregnancy | Preterm birth in babies exposed versus unexposed to lithium | The lithium-exposed cohort had a 36% prevalence of preterm delivery. In a cohort of 350 women, significantly more infants in the lithium-exposed group were born at <38 weeks of gestation (33%), compared with infants born to mothers with manic-depressive illness who did not receive lithium (13%) or with mothers without a bipolar disorder diagnosis (12%). Thus, the relative risk of premature delivery for women taking lithium during pregnancy is 2.54 times that for women with or without manic-depressive illness who are not receiving lithium during pregnancy. |
| Munk-Olsen 2018 (8) (cumulative data from six cohorts); included in meta-analysis | 22,124 | Denmark register-based cohort (1997–2012); Sweden register-based cohort (2005–2013); Canada register-based cohort (2002–2013); Netherlands clinical cohort; UK clinical cohort (2007–2013); U.S. clinical cohort (2004–2015) | Pregnancy (first trimester or any time) | Lithium-exposed group versus mood disorder reference group | Primary data from pregnant women and their children from six international cohorts based in the community and clinics. Lithium exposure was not associated with any of the predefined pregnancy complications or delivery outcomes. An increased risk for neonatal readmission within 28 days of birth was seen in the lithium-exposed group compared with the reference group (pooled prevalence, 27.5% [95% CI=15.8–39.1] compared with 14.3% [95% CI=10.4–18.2]; pooled adjusted odds ratio=1.62 [95% CI=1.12–2.33]). Lithium exposure during the first trimester was associated with an increased risk of major malformations (pooled prevalence, 7.4% [95% CI=4.0–10.7] compared with 4.3% [95% CI=3.7–4.8]; pooled adjusted odds ratio=1.71 [95% CI=1.07–2.72]), but for major cardiac malformations the difference was not significant (2.1% [95% CI=0.5–3.7] compared with 1.6% [95% CI=1.0–2.1]; pooled adjusted odds ratio=1.54 [95% CI=0.64–3.70]). |
| Efficacy: lithium versus nonlithium interventional studies | |||||
| Austin 1992 (36); included in the meta-analysis | 17 | Royal Edinburgh Hospital (Scotland) | Pregnancy and postpartum | Postpartum relapse in women using or not using lithium | Lower relapse rates for lithium-treated compared with non-lithium-treated women during postpartum (odds ratio=0.14, 95% CI=0–6.5). All relapsing women were admitted to hospital with moderate to severe episodes of mania, and all except two (in the untreated group) relapsed within 3 weeks of parturition. The average duration of inpatient stay was 7 weeks (range, 5–12 weeks), and four of six relapsing women from the untreated group were subsequently started on lithium prophylaxis. |
| Bergink 2012 (37); included in the meta-analysis | 41 | Peripartum Prevention Program, Department of Psychiatry, Erasmus Medical Center (Rotterdam, the Netherlands) | Pregnancy and postpartum | Relapse during pregnancy and postpartum in women using or not using lithium | Of the women with bipolar disorder (N=41), 24.4% relapsed during pregnancy, despite prophylaxis use by the majority throughout pregnancy. The postpartum relapse rate was highest in women with bipolar disorder who experienced mood episodes during pregnancy (60%). Patients with bipolar disorder require continuous prophylaxis throughout pregnancy and the postpartum period to reduce peripartum relapse risk. |
| Efficacy: lithium group only interventional study | |||||
| Rosso 2016 (49) | 18 | Psychiatric Unit of the Department of Neurosciences (University of Turin, Italy) | Pregnancy and postpartum | Rates of bipolar recurrence during pregnancy | Bipolar recurrences of any polarity during pregnancy occurred in 11.1% of the women. The results support the efficacy of lithium prophylaxis throughout pregnancy in lithium-responding women with bipolar I disorder. No serious side effects were noted for mother or baby. |
| Efficacy: retrospective cohort studies | |||||
| Viguera 2000 (7) | 101 | Perinatal and Reproductive Psychiatry Research Program, Massachusetts General Hospital, Boston, and Lucio Bini–Stanley Foundation Center for Mood Disorders Research, Cagliari, Sardinia | Pregnancy and postpartum | Recurrence rates in pregnant women versus nonpregnant women during rapid or gradual discontinuation of lithium, during pregnancy and postpartum | Rates of recurrence during the first 40 weeks after lithium discontinuation were similar for pregnant and nonpregnant women but then sharply increased postpartum. The risk was much lower with gradual discontinuation. Recurrence rates were similar for bipolar I and II disorders but were higher in patients with a history of four or more prior episodes of illness and for those who underwent rapid discontinuation of lithium. |
| Wesseloo 2017 (50) | 114 | Danish national registry | Pregnancy and postpartum | Postpartum recurrence of bipolar disorder in women using lithium versus women using lamotrigine | No difference was observed between lithium and lamotrigine in the prevention of severe postpartum episodes. |
Quality of the Included Studies
Meta-Analysis
| Outcome (Reference) | Articles (N) | k | Cases Among Lithium-Exposed Women | Lithium-Exposed Women, Overall | Cases Among Lithium-Unexposed Women | Lithium-Unexposed Women, Overall | Odds Ratiob | 95% CI | I2 (%) | p | NNHc | 95% CI | I2 (%) | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall exposure to lithium at any time during pregnancy compared with unexposed women (either with bipolar disorder or general-population controls) | ||||||||||||||
| Spontaneous abortion (2, 26) | 2 | 3 | 43 | 321 | 6 | 968 | 3.77 | 1.15–12.39 | 86.55 | 0.03 | 15 | 8–111 | 56.17 | 0.03 |
| Preterm birth (2, 8, 26–29) | 6 | 13 | 144 | 1,084 | 2,047 | 22,611 | 1.42 | 0.98–2.06 | 60.61 | 0.07 | 23 | 11–100 | 53.82 | 0.05 |
| Low birth weight (2, 8, 26) | 3 | 9 | NA | 980 | NA | 22,258 | 0.99 | 0.84–1.19 | 0 | 0.99 | 143d | 38 –83 | NAd | 0.48 |
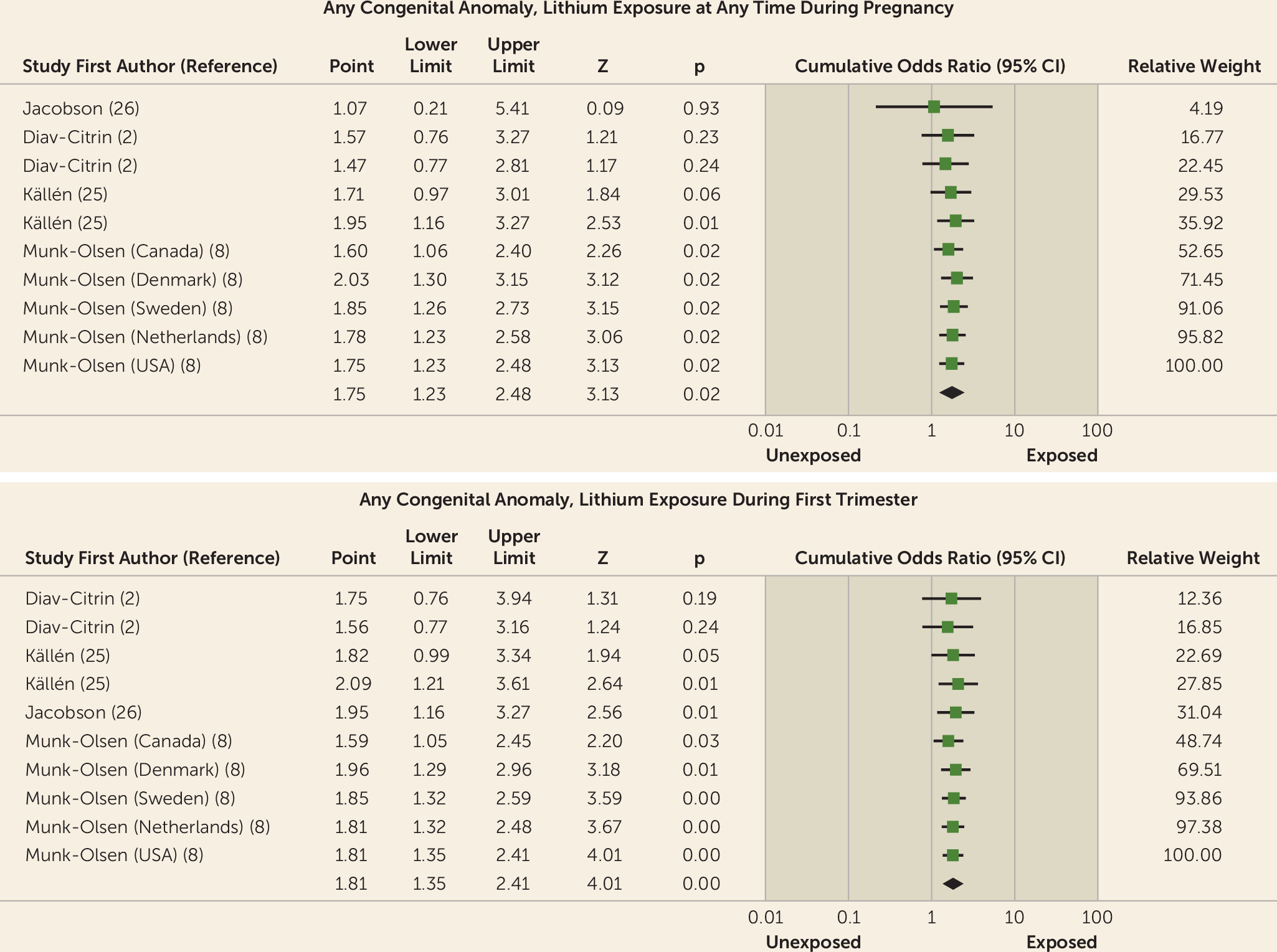
| Any congenital anomaly (2, 8, 25, 26) | 4 | 11 | 69 | 1,195 | 889 | 22,105 | 1.75 | 1.23–2.48 | 25.96 | <0.01 | 33 | 22–77 | 6.61 | <0.01 |
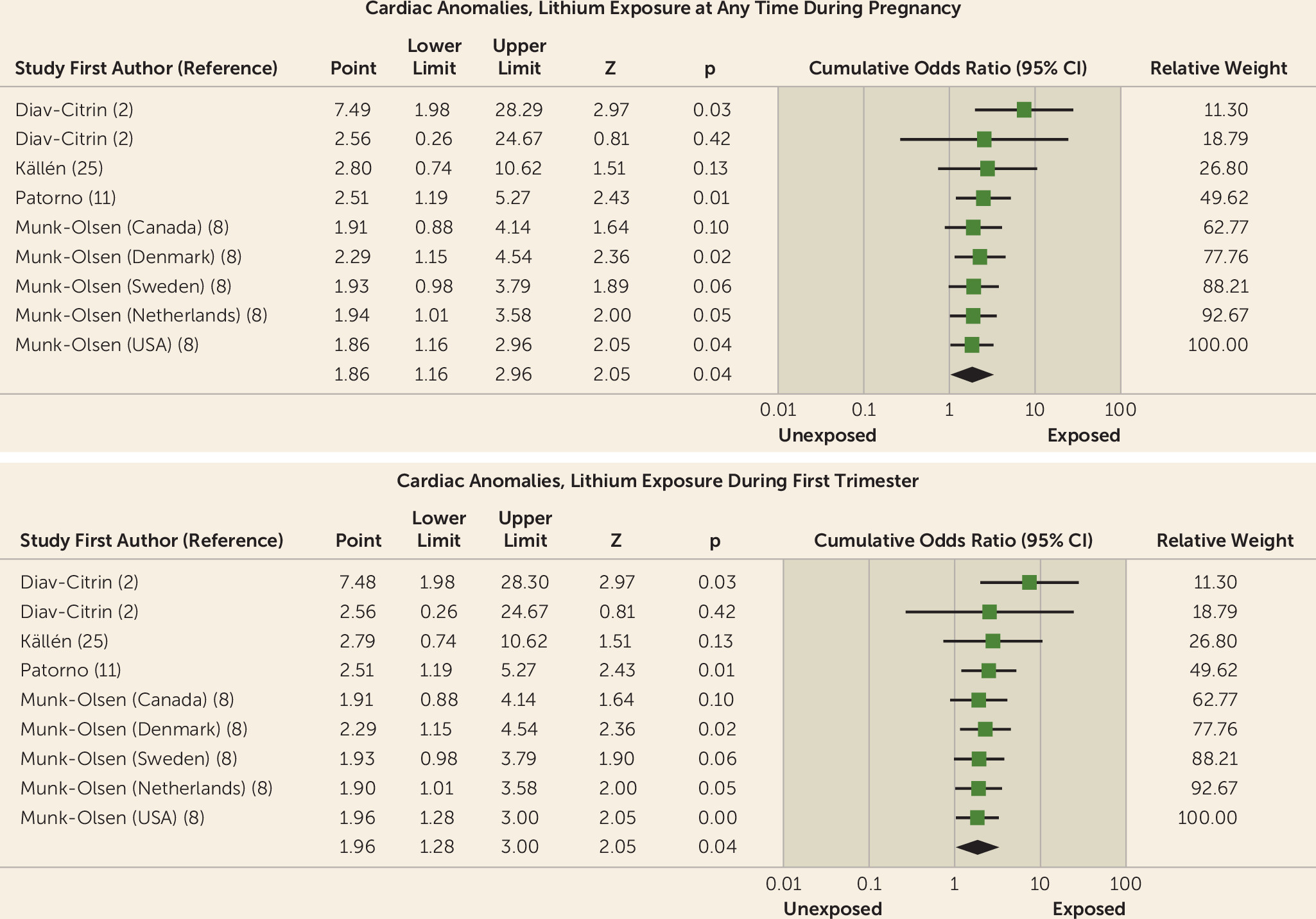
| Cardiac anomaly (2, 8, 11, 25) | 4 | 12 | 43 | 1,508 | 15,604 | 1,346,967 | 1.86 | 1.16–2.96 | 40.16 | <0.01 | 71 | 48–167 | 4.62 | <0.01 |
| Exposure to lithium at any time during pregnancy compared with unexposed general population | ||||||||||||||
| Preterm birth (2, 27) | 2 | 2 | 19 | 151 | 42 | 694 | 2.22 | 0.99–4.97 | 6.68 | 0.05 | 18 | 7–29 | 22.27 | 0.22 |
| Any congenital anomaly (2, 25) | 2 | 2 | 15 | 182 | 24 | 821 | 2.03 | 1.03–3.99 | 0 | 0.04 | 22 | 12–200 | 0 | 0.03 |
| Cardiac anomaly (2, 11, 25) | 3 | 2 | 26 | 815 | 15,256 | 1,323,776 | 3.99 | 1.19–13.43 | 63.17 | 0.03 | 37 | 19–1000 | 46.36 | 0.04 |
| Exposure to lithium at any time during pregnancy compared with unexposed patients with bipolar disordere | ||||||||||||||
| Spontaneous abortion (2, 26) | 2 | 2 | 43 | 321 | 18 | 220 | 2.46 | 0.56–10.77 | 82.09 | 0.23 | 24 | 9–42 | 35.61 | 0.21 |
| Preterm birth (2, 8, 26–29) | 6 | 11 | 144 | 1,084 | 2005 | 21,917 | 1.34 | 0.89–2.01 | 62.85 | 0.16 | 26 | 11–62 | 61.46 | 0.16 |
| Low birth weight (2, 8, 26) | 3 | 8 | NA | 980 | NA | 21,547 | 1.07 | 0.85–1.34 | 0 | 0.56 | 143d | 38–83 | NAd | 0.48 |
| Any congenital anomaly (2, 8, 25, 26) | 4 | 9 | 69 | 1,013 | 865 | 21,284 | 1.75 | 1.21–2.52 | 15.35 | <0.01 | 38 | 20–333 | 29.31 | 0.03 |
| Cardiac anomaly (2, 8, 11, 25) | 4 | 9 | 42 | 1,508 | 348 | 23,191 | 1.59 | 0.91–2.77 | 35.44 | 0.10 | 91 | 50–500 | 0 | 0.01 |
| Efficacy associated with lithium exposure during pregnancy/peripartum | ||||||||||||||
| Relapse, postpartum, any mood episode (36, 37) | 2 | 2 | 4 | 35 | 7 | 13 | 0.16 | 0.03–0.89 | 55.81 | 0.04 | —f | 1–12 | 52.76 | 0.12 |
Spontaneous abortion.
| Outcome | k | Cases Among Lithium-Exposed Women | Lithium-Exposed Women, Overall | Cases Among Lithium-Unexposed Women | Lithium-Unexposed Women, Overall | Odds Ratiob | 95% CI | I2 (%) | p | NNHc | 95% CI | I2 (%) | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall exposure to lithium during the first trimester compared with unexposed women (either bipolar disorder or general-population controls) | |||||||||||||
| Spontaneous abortion (2, 26) | 3 | 43 | 321 | 61 | 968 | 3.77 | 1.15–12.39 | 86.55 | 0.03 | 15 | 8–111 | 56.17 | 0.03 |
| Preterm birth (2, 26) | 3 | 24 | 269 | 54 | 890 | 1.72 | 0.96–3.08 | 60.61 | 0.07 | 29 | 11–48 | 51.97 | 0.12 |
| Low birth weight (2, 26) | 3 | NA | 269 | NA | 890 | 1.01 | 0.80–1.28 | 9.13 | 0.99 | NA | NA | NA | |
| Any congenital anomaly (2, 8, 25, 26) | 10 | 65 | 1123 | 889 | 22,105 | 1.81 | 1.35–2.41 | 0 | <0.001 | 32 | 21–77 | 8.80 | 0.001 |
| Cardiac anomaly (2, 8, 11, 25) | 11 | 42 | 1,436 | 15,604 | 1,346,967 | 1.96 | 1.28–3.00 | 29.92 | <0.01 | 71 | 48–143 | 11.8 | <0.001 |
| Exposure to lithium during the first trimester compared with the unexposed general population | |||||||||||||
| Any congenital anomaly (2, 25) | 2 | 15 | 182 | 24 | 821 | 2.03 | 1.03–3.99 | 0 | 0.04 | 22 | 12–200 | 0 | 0.03 |
| Cardiac anomaly (2, 11, 25) | 3 | 26 | 815 | 15,256 | 1,323,776 | 3.99 | 1.19–13.43 | 63.17 | 0.03 | 37 | 19–1000 | 46.36 | 0.04 |
| Exposure to lithium during the first trimester compared with unexposed patients with bipolar disorderd | |||||||||||||
| Spontaneous abortion (2, 26) | 2 | 43 | 321 | 18 | 220 | 2.46 | 0.56–10.77 | 82.09 | 0.23 | 24 | 9–42 | 35.61 | 0.21 |
| Preterm birth (2, 26) | 2 | 24 | 269 | 13 | 207 | 1.17 | 0.56–2.44 | 0 | 0.68 | 25 | 23–31 | 0 | 0.86 |
| Low birth weight (2, 26) | 2 | NA | 269 | NA | 207 | 1.17 | 0.85–1.61 | 0 | 0.34 | NA | NA | NA | |
| Any congenital anomaly (2, 8, 25, 26) | 8 | 65 | 941 | 865 | 21,284 | 1.75 | 1.21–2.98 | 15.35 | <0.01 | 37 | 19–333 | 32.41 | 0.03 |
| Cardiac anomaly (2, 8, 11, 25) | 8 | 41 | 1,436 | 348 | 23,191 | 1.75 | 1.08–2.84 | 19.99 | 0.02 | 83 | 48–333 | 0 | 0.01 |
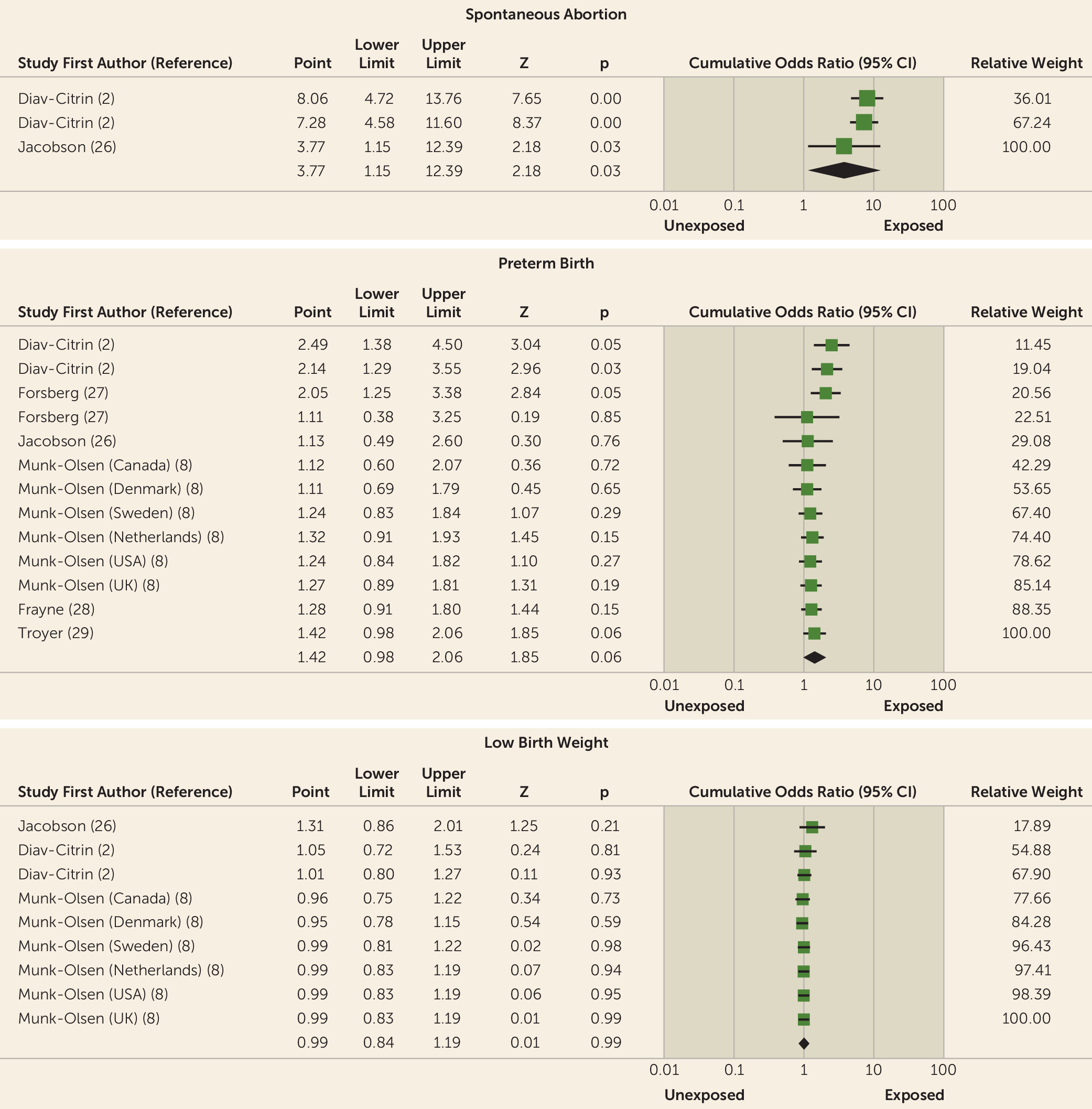
Preterm birth and low birth weight.
Any congenital anomaly.
Cardiac anomalies.
Relapse.
Discussion
Footnote
Supplementary Material
- View/Download
- 254.44 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Author Contributions
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

