Exposure to traumatizing events is often followed by dissociative experiences (
1), such as amnesia, flashbacks, numbing, depersonalization, derealization, passive influence phenomenon, and identity disturbances, suggesting that in acutely threatening contexts, dissociation often serves a defensive or coping function (
2). It has been proposed that in the context of recovery from trauma, dissociative experiences provide psychological distance (
3).
And yet long-term predisposition to dissociative experiences can affect an individual’s ability to function. Dissociation is a frequent consequence in cases of trauma that are especially severe, chronic, or occurring during key periods of brain development when emotional systems are highly sensitive to experience (
4). Consequently, the presence or absence of dissociative experiences serves as an important indicator of clinical severity across many posttraumatic syndromes. These include posttraumatic stress disorder (PTSD) (
5) as well as some syndromes in which dissociation represents a core pathology, such as dissociative identity disorder (
6).
Despite the clinical importance of dissociative symptoms in characterizing posttraumatic syndromes and recent foundational work beginning to define an associated neurobiology (
7), there are no objective tests available to corroborate subjective reports of dissociation. This hampers the ability of individuals experiencing dissociation to receive appropriate care, including accurate diagnosis, prognosis, and treatment selection. Moreover, the reliance on subjective reports has limited our understanding of the neurobiology of dissociative experiences, since the wide heterogeneity in whether and how individuals report what they have experienced may lead to high levels of unexplained within-class variance.
Addressing this issue by developing methods to corroborate reports of severe dissociation would therefore advance the field substantially, leading to a better understanding of the biological underpinnings of dissociation and possibly new avenues for treatment. Brain-based methods for objective symptom corroboration are attractive as symptom biomarkers because they do not rely on any outward manifestation of symptoms or physiology, which could in principle be obscured by an individual aware of those manifestations.
Many studies have documented evidence of brain changes associated with a predisposition to dissociative experiences. For instance, evidence from seed-based connectivity analyses while participants with PTSD and its dissociative subtype are at rest suggests entrenched patterns of emotion and arousal overmodulation in depersonalization and derealization (
8,
9), that is, cortical overmodulation of limbic structures dominates processing. Evidence from individuals with dissociative identity disorder demonstrates neurobiological patterns similar to those seen in the dissociative subtype of PTSD when individuals with dissociative identity disorder are in a numb and detached state (
10). Reinders and colleagues (
11) have also successfully used machine learning to distinguish between individuals with dissociative identity disorder and healthy control subjects based on their brain morphology. Together this work indicates that the subjective experience of dissociation is associated with measurable brain differences at the group level and structural brain differences at the individual level; however, whether or not these changes are sufficiently sensitive and robust to allow for individual-level estimation of dissociation severity based on brain function has not yet, to our knowledge, been tested. In aggregate, these studies indicate that it may be possible to develop methods based on changes in brain structure and function to support current or recently reported dissociative experiences, providing at least a path toward a stable symptom biomarker.
To address this gap and determine the extent to which a noninvasive, brain-derived biomarker based on cortical network connectivity could provide an objective means to corroborate a report of dissociation, we conducted a cross-sectional observational study of women receiving care for posttraumatic psychopathology, each manifesting a different severity of dissociative symptoms, using an extended functional MRI (fMRI) protocol. To determine whether these fMRI data were sufficient to estimate individual-level dissociation severity, we employed a novel machine-learning approach using individual participant brain connectivity measures (
12). We employed a brain connectivity analysis approach in which cortical functional boundaries are defined at the individual level, prior to assessing connectivity between regions. This approach has demonstrated substantial improvements in the sensitivity and specificity of connectivity-based symptom estimation in diverse symptom domains, including obsessive-compulsive disorder, schizophrenia spectrum disorders, and bipolar disorder (
13,
14).
Methods
Participants
Participants were 75 women seeking inpatient, partial, residential, or outpatient treatment at a psychiatric hospital in the northeastern United States. All participants had a history of interpersonal childhood maltreatment, current PTSD, and various levels of dissociative symptoms, including some with co-occurring dissociative identity disorder.
Participants were excluded if they had any absolute or relative standard contraindications to MRI. Other exclusion criteria included a history of neurological conditions, history of head injury resulting in a loss of consciousness for longer than 5 minutes, a current alcohol or substance use disorder within the past month, and a history of psychotic spectrum disorders. Data from 65 participants were retained for subsequent analysis after application of these exclusion criteria and imaging quality control, described below. These participants’ demographic and clinical characteristics are summarized in
Table 1. The Massachusetts General Brigham Human Research Affairs Institutional Review Board approved all procedures, which were performed in accordance with human subject guidelines and regulations. All participants provided written informed consent after treating clinicians had assessed the participant’s clinical competence to provide informed consent.
Diagnostic and Symptom Measures
The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) (
15) was used to diagnose PTSD, and the Structured Clinical Interview for DSM-IV Dissociative Disorders (
16) was used to diagnose dissociative disorders. The CAPS-5 total PTSD symptom severity score was also used to control for PTSD symptom severity in our models. This score is a measure of PTSD severity across all symptom domains, and ranges from 0 to 100. In our sample, the CAPS-5 total symptom severity score displayed good internal consistency (Cronbach’s alpha=0.83).
Our primary measure of interest was the Multidimensional Inventory of Dissociation (
17), a comprehensive self-report instrument of pathological dissociative symptoms. We focused on predicting the instrument’s severe dissociation score in our model. This score is an indication of how many of the dissociative symptom items reach clinical levels of significance. Scores range from 0 to 168. In our sample, the Multidimensional Inventory of Dissociation displayed excellent internal consistency (Cronbach’s alpha=0.99). Additionally, we used the Childhood Trauma Questionnaire (CTQ) (
18) to evaluate the frequency of childhood maltreatment. The CTQ total score ranges from 25 to 125. In our sample, the CTQ displayed excellent internal consistency (Cronbach’s alpha=0.94).
Neuroimaging Procedures
MRI scanning was performed in a 3-T Tim Trio scanner (Siemens Healthcare, Erlangen, Germany) using the vendor-supplied 12-channel phased-array head coil and standard T
2*-weighted echo-planar imaging (TR=3000 ms, TE=30 ms, flip angle=85°, 3×3×3 mm voxels) for blood-oxygen-level-dependent (BOLD) fMRI. Participants performed a series of tasks during BOLD imaging: a multisource interference task (396 seconds) (
19), a masked faces task (450 seconds) (
20), and a “rest” task, during which participants were instructed to lie still with their eyes open (372 seconds).
Imaging Data Preprocessing and Analysis
Resting-state and task-based fMRI data were processed in the same way, using procedures described elsewhere (
21). Briefly, processing included discarding the first four volumes, slice timing correction, motion correction, bandpass filtering, motion regression, whole brain signal regression, and ventricular and white matter regression. The data of different tasks (including the “rest” task) were concatenated within each subject to increase the amount and reliability of data per subject. Subjects with head motion greater than 0.2 mm and temporal signal-to-noise ratio <100 were excluded from further analyses. Functional regions of interest were localized in each individual. First, a fine-grained and population-level parcellation with 92 regions of interest (see Figure S1A in the
online supplement) across the whole brain was created on the basis of a sample of 1,000 subjects from the Genomic Superstruct Project (
22). Briefly, we split the cerebral cortex into five lobes—frontal, parietal, temporal, occipital, and motor lobes—according to the Desikan-Killiany atlas (
23), and then applied a k-means clustering approach to segment each lobe into multiple subareas based on the functional connectivity profile. The functional connectivity profile was estimated as Pearson’s correlation between the time series of each vertex and the other 1,175 vertices, which were uniformly sampled in FreeSurfer fsaverage6 space. Second, we applied our previously reported iterative parcellation strategy to derive an individual-level parcellation of each lobe (for more details, see references
12,
24). Using this procedure, a total of 92 individualized homologous functional regions of interest were localized in the 65 participants in our data set. These functional regions of interest demonstrated substantial interindividual variability in size and position across individuals (see Figure S1B in the
online supplement).
Next, we estimated symptom-connectivity associations (
12,
13). In summary, we derived connectivity estimates by individually defining homologous functional nodes (i.e., regions) for each participant and then computed edge weights across the entire cortical connectivity matrix. Subsequently, a support vector machine for regression (SVR) algorithm (L2-regularized L2-loss SVR model with default parameters) implemented in the LIBLINEAR package (
https://www.csie.ntu.edu.tw/∼cjlin/liblinear/) was used to estimate participants’ Multidimensional Inventory of Dissociation severe dissociation scores, using a leave-one-subject-out cross-validation (LOOCV) approach. Features that were significantly (p<0.01 or p<0.005) correlated with the Multidimensional Inventory of Dissociation severe dissociation scores were selected to train the SVR model in each LOOCV. Importantly, covariates including motion, age, childhood trauma severity, and PTSD symptom severity were regressed from both the brain features and the measured severe dissociative symptom scores. This approach yields the optimal combination of brain network edges that can be associated with symptom scores, if such a solution exists for the desired outcome measure.
As mentioned above, individualized functional regions demonstrated marked intersubject variability in size, which can be related to individual differences in behavior (
12,
25–
27). We further trained the SVR model described above to investigate whether the size of the functional regions was related to the severe dissociative symptom scores.
Permutation testing was performed to create a distribution of random SVR models to determine whether the prediction of severe dissociative symptom scores exceeded chance levels. The measured symptom scores were randomly reshuffled 1,000 times among the subjects, and the prediction procedures were repeated each time. The permutation p value was calculated as the percentage of permutations that yielded a prediction-measured correlation value higher than the prediction-measured correlation based on the real data. Weights/contributions of the features (functional connection or size of functional regions) in the prediction were estimated as the absolute value of the regression coefficients of corresponding features in the SVR model. Selected functional connections varied slightly within each LOOCV. Because of this variation, we only displayed “consensus features” in our figures. Consensus features were those that were common to LOOCVs as important features for severe dissociation score prediction.
Finally, the prediction analyses based on individualized functional connectivity were repeated using k-fold cross-validation with the traditionally suggested fivefold model. Specifically, we trained the model using 80% of the subjects and tested the model in the remaining 20% of the subjects. The cross-validation was repeated 100 times, and the mean prediction accuracy r, r-squared, and mean squared error (i.e., how much predicted values deviate from true values) were reported to estimate the prediction performance.
Results
Individually Specified Functional Connectome Tracks of Severe Dissociation Symptoms
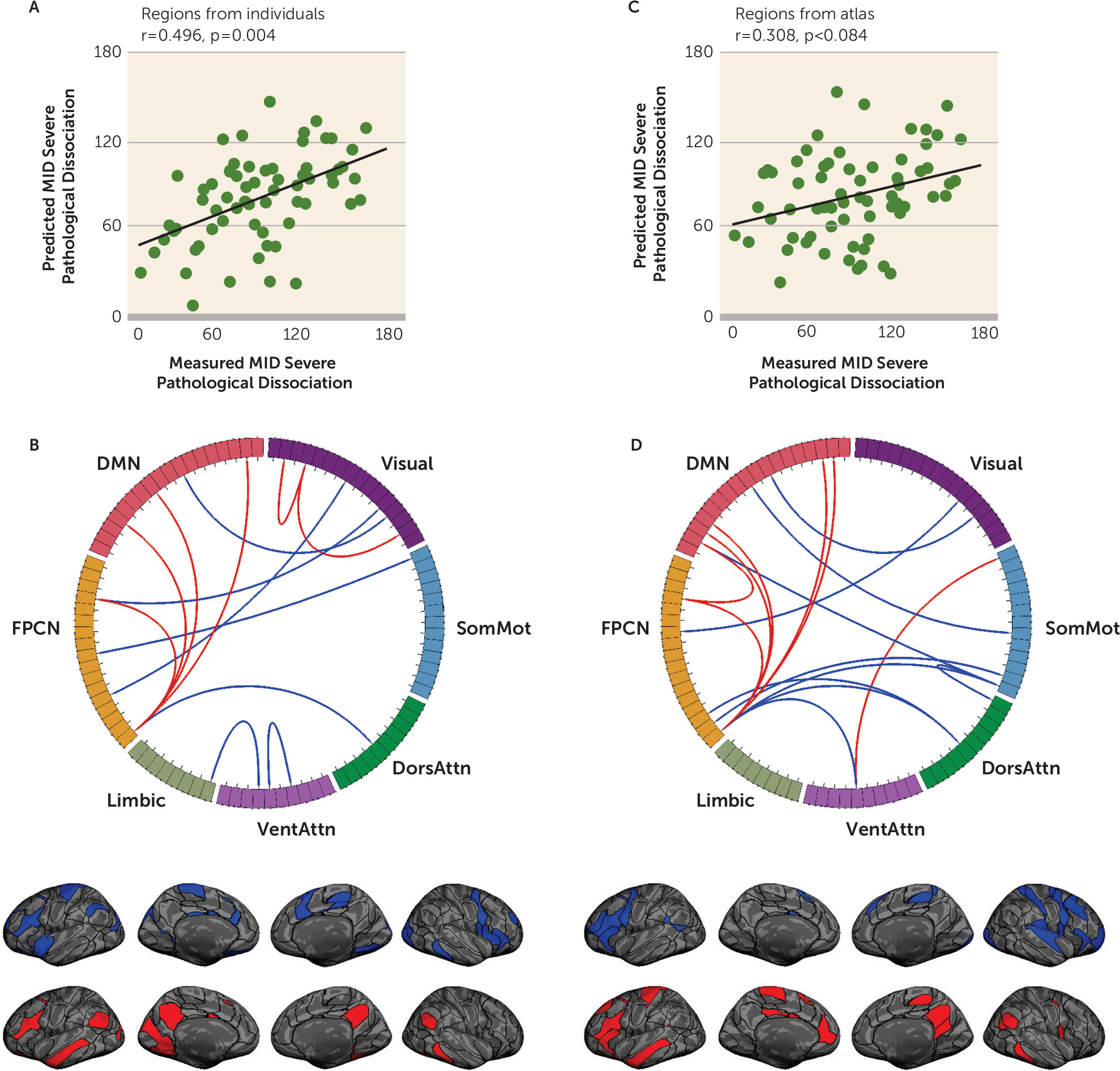
To determine whether individually specified functional connectivity tracked with the severe dissociation scores, we trained SVR models to estimate the Multidimensional Inventory of Dissociation severe dissociation score from each of the individual participants. We found that the severe dissociative symptom score was robustly estimated by a set of functional connections, with a significant correlation between estimated and observed scores among the 65 patients (
Figure 1A) (r=0.496, p=0.004, 1,000 permutation tests). We further calculated the partial correlation between the predicted and observed symptoms, while controlling for head motion. We found that controlling for head motion had almost no effect on the correlations (Pearson’s correlation, r=0.479, p<0.001; see Figures S1 and S2 in the
online supplement for further covariate explorations). Depending on the LOOCV model, the feature number contributing to the estimation of severe dissociation scores ranged from 30 to 43. Connections that contributed most to the estimation of severe dissociation scores mainly involved the frontoparietal control network and default mode network (
Figure 1B).
For comparison, the SVR analysis was repeated using functional connectivity among the corresponding functional regions identified in the group-level atlas (
21) (see Figure S3A in the
online supplement). The correlation between the predicted and observed Multidimensional Inventory of Dissociation severe dissociation score was greatly reduced (p<0.006, z=2.52, Steiger’s z test; prediction accuracy r=0.308, p=0.084, 1,000 permutation tests) (
Figure 1C). The number of features contributing to the severe dissociation estimation ranged from 33 to 52, depending on the LOOCV model. The most predictive connections from the atlas again involved the frontoparietal control and default mode networks, although the prediction was weaker (
Figure 1D). We repeated the analysis using another prominent group-level atlas (
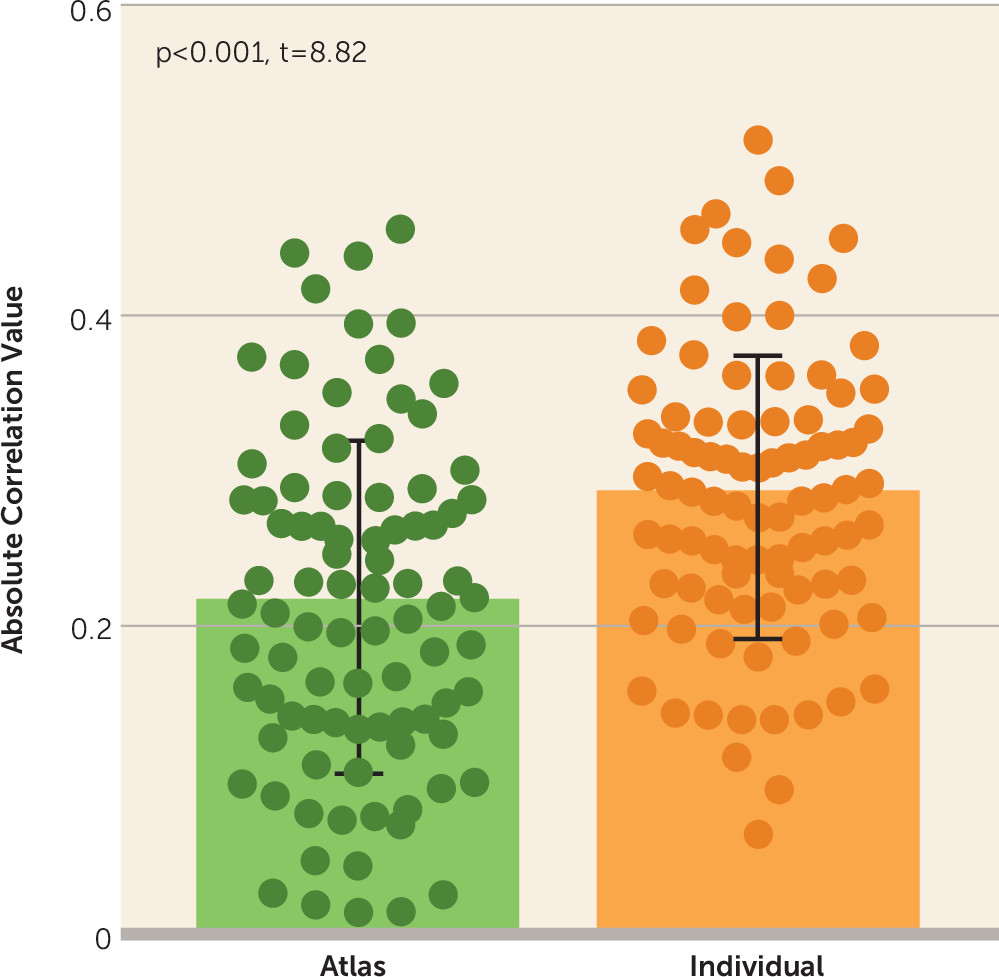
28), which consisted of 360 regions, and this analysis tended to yield similar results (p=0.049, z=1.65, Steiger’s z test; prediction accuracy r=0.351, p=0.003 permutation test). Importantly, we found that the connections defined by group-level regions were less correlated with symptom scores (
Figure 2) (p<0.001, t=8.82, paired t test) compared with the same connections defined by individualized regions. This indicated that the symptom-related connections were obscured by the group-level atlas, impairing the prediction of symptoms.
As a final step, we repeated the prediction analysis based on the individual-specified connectivity using fivefold cross-validation and found that our findings were robust. We showed that individually defined regions are superior across different objective indices and cross-validations (see Table S1 in the online supplement).
Estimating Within-Network and Between-Network Functional Connectivity
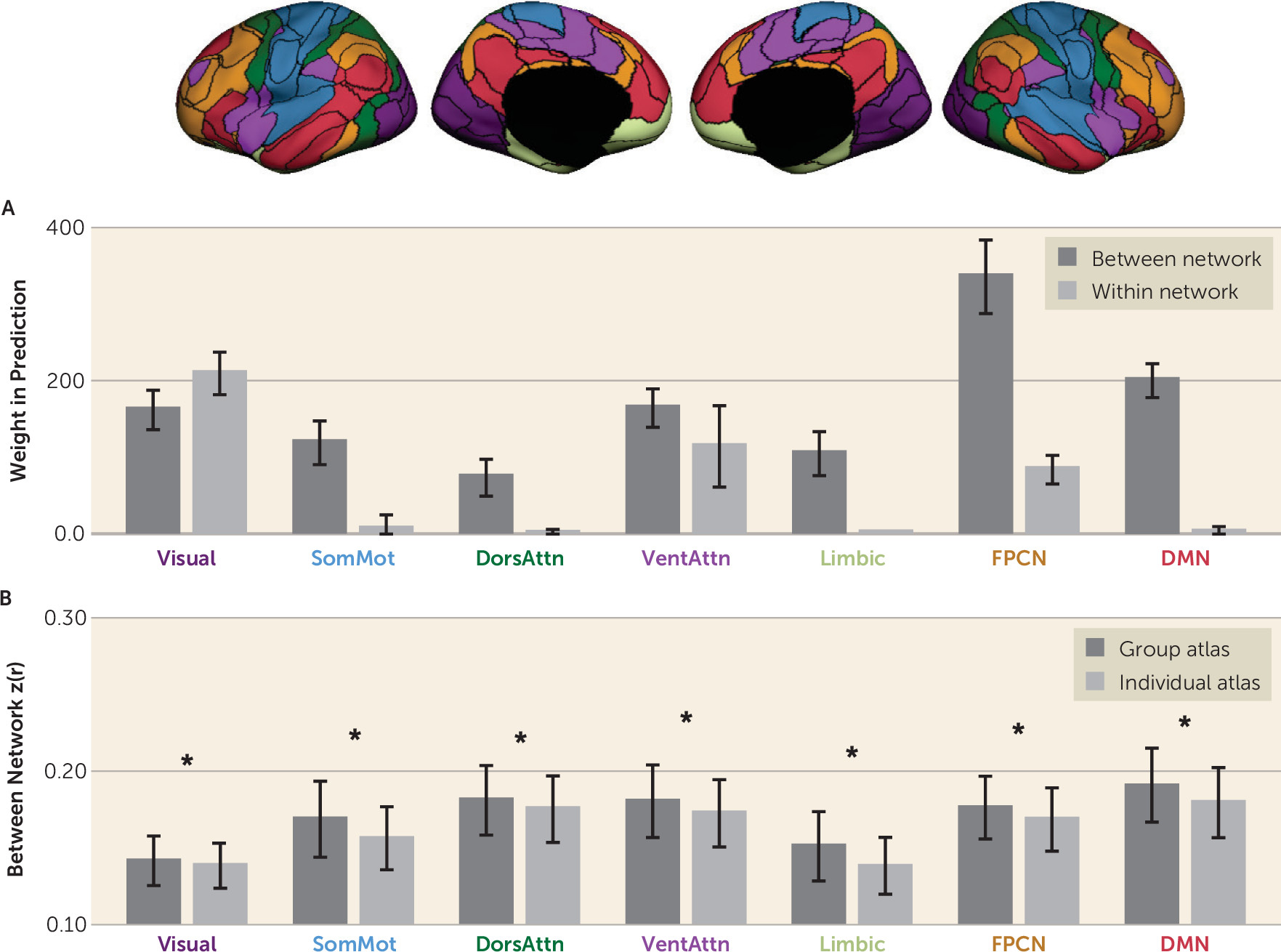
Functional connections were separated into within-network and between-network connections according to whether they connected two regions in the same network or different networks (
Figure 3). Within-network and between-network connectivity values were estimated for each participant. To compute the within-network connectivity of a specific network, we averaged the connectivity values of all region pairs within the network. To compute the between-network connectivity of a specific network, we averaged the connectivity values of all region pairs that involved a region within the network and a region outside the network. We found that the connections contributing to the symptom estimation were mostly between-network connections that involved the visual, frontoparietal control, and default mode networks (
Figure 3A). Figure S4 in the
online supplement presents the weight distribution for both between and within networks divided by positive and negative direction.
We then investigated how between-network connectivity was changed by the subject-specific functional regions and found that the absolute values of between-network connections were significantly reduced (average decrease of 5.15%) when regions of interest were individually specified compared with atlas defined (
Figure 3B). Intriguingly, although the absolute values of between-network connectivity were significantly reduced, they yielded better symptom estimates, suggesting that between-network connectivity may be more accurately quantified when functional regions are localized in individuals.
Complementary Information From Size and Functional Connectivity of the Individually Specified Regions of Interest for Predicting Severe Dissociative Symptom Scores
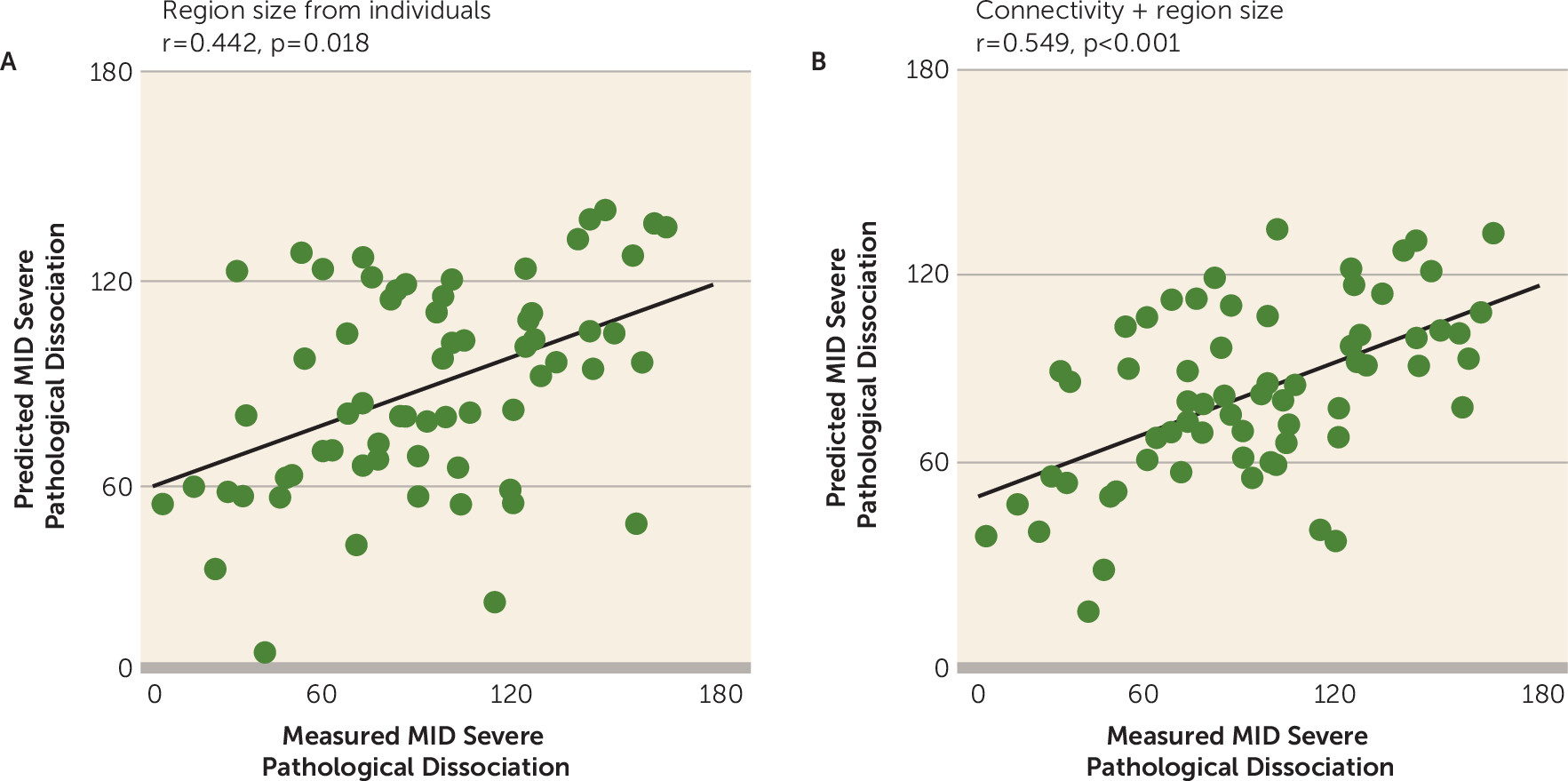
Functional connectivity studies have mostly focused on connectivity strength among brain regions, but rarely on the topography of the functional regions because no variable size was provided by atlas regions. Here, we examined whether the size of the individualized functional regions is behaviorally relevant. We found that the size of the individualized regions was predictive of symptom scores (r=0.442, p=0.018, permutation test) (
Figure 4A). Specifically, we observed a mild negative correlation (r=−0.277, p=0.031) between Multidimensional Inventory of Dissociation score and size of the ventral attention network but a positive correlation between Multidimensional Inventory of Dissociation score and size of the somatomotor network (r=0.271, p=0.035). The results indicate that the size of the functional regions provides useful information for the prediction of symptom scores. To further determine whether the size of the functional regions provides nonredundant information for functional connectivity in the prediction of symptom scores, we repeated the SVR analysis using features from the combination of region size and functional connections. We found that the severe dissociative symptom scores were better predicted in a combined model including region size and functional connections compared with either one of the single features by themselves (r=0.549, p<0.001, permutation test) (
Figure 4B).
Discussion
Despite foundational work on the neural basis for trauma-related dissociation, the field has yet to produce a clinical test, brain-based or otherwise, to corroborate subjective symptom reports and provide an objective assay documenting presence of or predisposition to severe dissociative symptoms. In the present work, we set out to discover whether a mapping exists between abnormalities in large-scale brain network connectivity and patterns of dissociative experiences that can be discriminated from patterns associated with experiencing other common posttraumatic symptoms (e.g., hyperarousal, nightmares). We used a measure of severe pathological dissociation to characterize tendency toward severe dissociation and tested whether we could estimate this score at the individual level from weighted, individualized functional connectivity estimates, after controlling for motion, age, childhood trauma, and PTSD symptom severity. We demonstrated that our models successfully predicted severe dissociative symptom scores, well above chance levels. Because our model controlled for childhood trauma and PTSD symptom severity, this suggests that trauma-related dissociation has neurobiological substrates that are distinct from PTSD and childhood trauma load.
Patterns of Network Connectivity Driving the Model
Machine learning is a technique used to produce prediction models, and it does not necessarily follow that high weights in our model mean that those network connections are more important for dissociation; instead, a high weight in the model means only that this connection is important in the regression model. Therefore, the main conclusion from our work is that aberrant network connectivity is associated with dissociative symptom estimation in our model.
Although this method is not intended to make statistical inferences about the biology of dissociation, it is nonetheless worth speculating that perhaps dissociative experiences are dependent on connections between regions in the default mode and frontoparietal control networks. The default mode network facilitates internally oriented attention often comprising past and future thinking and emotional and self-referential processing (
29,
30). The frontoparietal control network is involved in problem solving, working memory–related tasks, and decision making (
31). There is also evidence to suggest that the frontoparietal control network pairs with the default mode network to facilitate internally oriented, goal-directed cognition, that is, problem solving to accomplish one’s goals (
32). While it is speculative that these networks are more important to dissociation, our results suggest that various regions in the frontoparietal control and default mode networks are more likely to be active at the same time, the more severe the dissociative symptoms. This result may imply a dominance of internally oriented goal-directed cognition in individuals with high levels of dissociation. Given histories of interpersonal childhood abuse, individuals may have learned early on to rely on internal problem solving because caregivers were unreliable.
Limitations
Our interpretations are constrained by several important limitations. Participants were taking various forms of psychiatric medication, which our sample was insufficiently powered to address. Also, while we validated the main findings using fivefold cross-validation, we did not replicate our findings in an independent data set. This may affect the generalizability of our findings. Finally, in this cross-sectional study, we did not attempt to resolve whether brain connectivity changes associated with a predisposition to dissociative experiences is a trait-like or state-like effect. We also did not measure state dissociation in the scanner. Thus, it remains unclear whether individuals who experience changes in or recover from the severity of dissociation (e.g., with treatment) would manifest changes in the relevant functional connections we identified here. Future work will seek to address this issue and other related potential clinical confounders by following individuals longitudinally to observe how the neural systems affected by complex dissociative disorders may respond to treatment, potentially revealing new therapeutic targets as well as advancing our understanding of the basic neurobiology underlying recovery from trauma.
Clinical Implications and Significance
Our work has contributed to the growing body of literature demonstrating a brain basis for trauma-related dissociation. Biological evidence is particularly compelling regarding the legitimacy of psychiatric symptoms. Increased awareness and acceptance of dissociative symptoms may motivate patients to seek assessment and care, medical practitioners to provide adequate care, and insurance providers to cover treatment. Better understanding of the biological correlates of trauma-related dissociation may also inform treatment approaches and the identification of psychopharmacological targets for further research.
A further potential use for capturing brain-based measures of dissociation, rather than assessing these symptoms with a self-report measure, is in assessments in individuals who are unable to effectively use the self-report (e.g., they unconsciously or consciously minimize or exaggerate their symptoms) or in situations where objective corroborating evidence is requested (e.g., court proceedings). Our work represents a first step toward building models of dissociation that could be used in these ways.
Finally, recent research demonstrates that one can reliably identify individual differences in mental health issues from unique patterns of functional brain connectivity—similar to a fingerprint (
33). Our work represents a foundational step toward building a functional connectivity fingerprint of trauma-related dissociation that may eventually contribute to improved diagnostic and biomarker tools to better understand the neural activity of people with these symptoms and assess the effects of treatment on an individual basis.
Acknowledgments
Dr. Kaufman was supported by NIMH grant R21MH112956, the O’Keefe Family Foundation, the Trauma Scholars Fund, the Barlow Family Fund, and the Julia Kasparian Fund for Neuroscience Research at McLean Hospital. Dr. Liu was supported by NIH grants 1R01NS091604 and P50MH106435, Beijing Municipal Science and Technology Commission grant Z161100002616009, and National Natural Science Foundation of China grants 81790650 and 81790652. Dr. Ressler was supported by the Frazier Foundation Grant for Mood and Anxiety Research. Dr. Lebois was supported by NIMH grants F32MH109274 and K01MH118467. Dr. Wang was supported by NIH grant K01MH111802.
The authors thank the participants for making this research possible, the staff of the Hill Center for Women and Proctor House II, and Scott Rauch, M.D., for discussion and feedback.