Alcohol Exposure Findings
To our knowledge, this is the largest examination of prenatal alcohol exposure and psychological, behavioral, and neurodevelopmental outcomes in preadolescence. The estimated total number of drinks consumed during pregnancy ranged from 0 to 90 following outlier conversion. This alcohol dose is relatively low, and the parent-reported exposure patterns prevalent in the ABCD cohort are more typical and reflective of the general population than those investigated in previous studies of fetal alcohol spectrum disorder (
2).
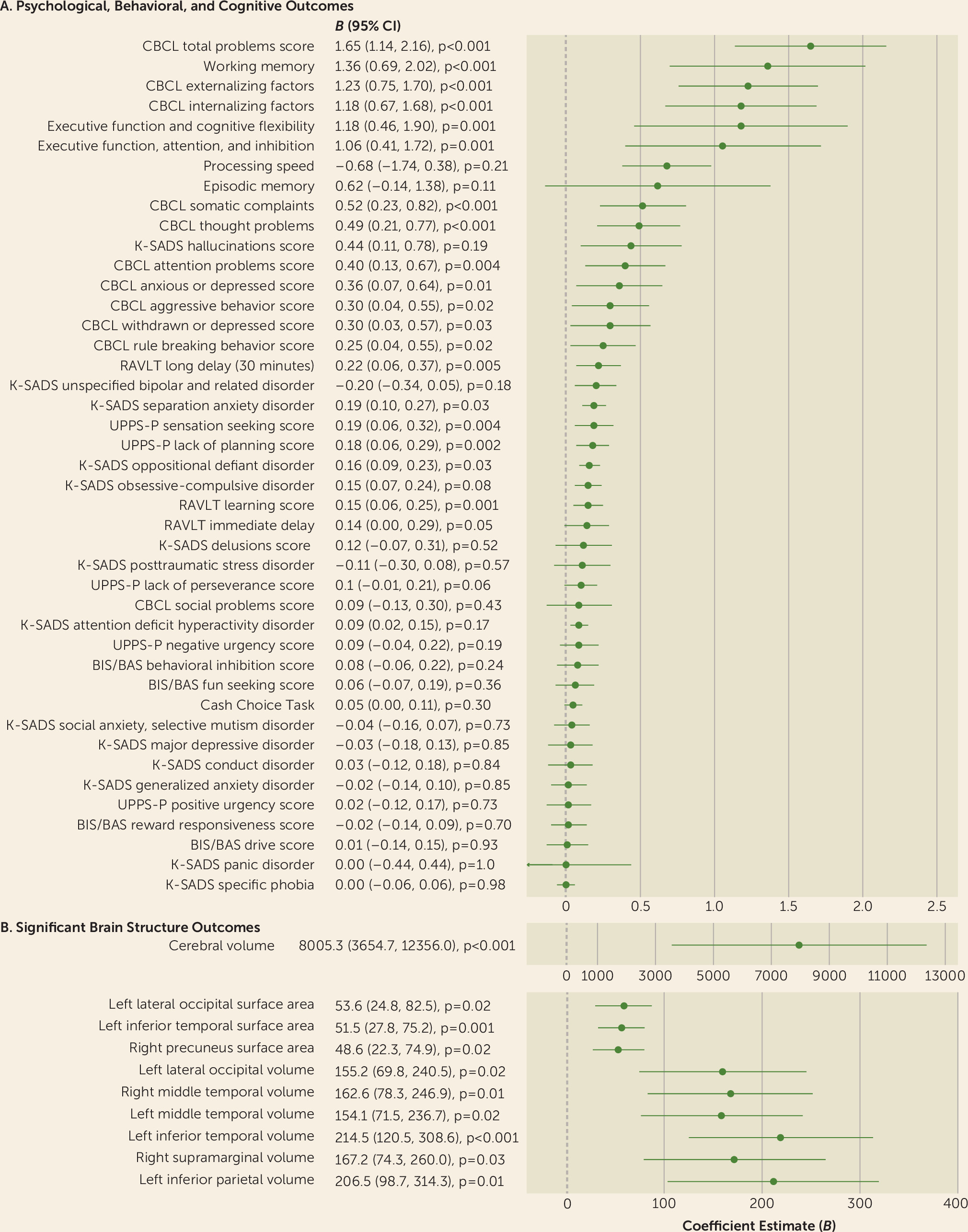
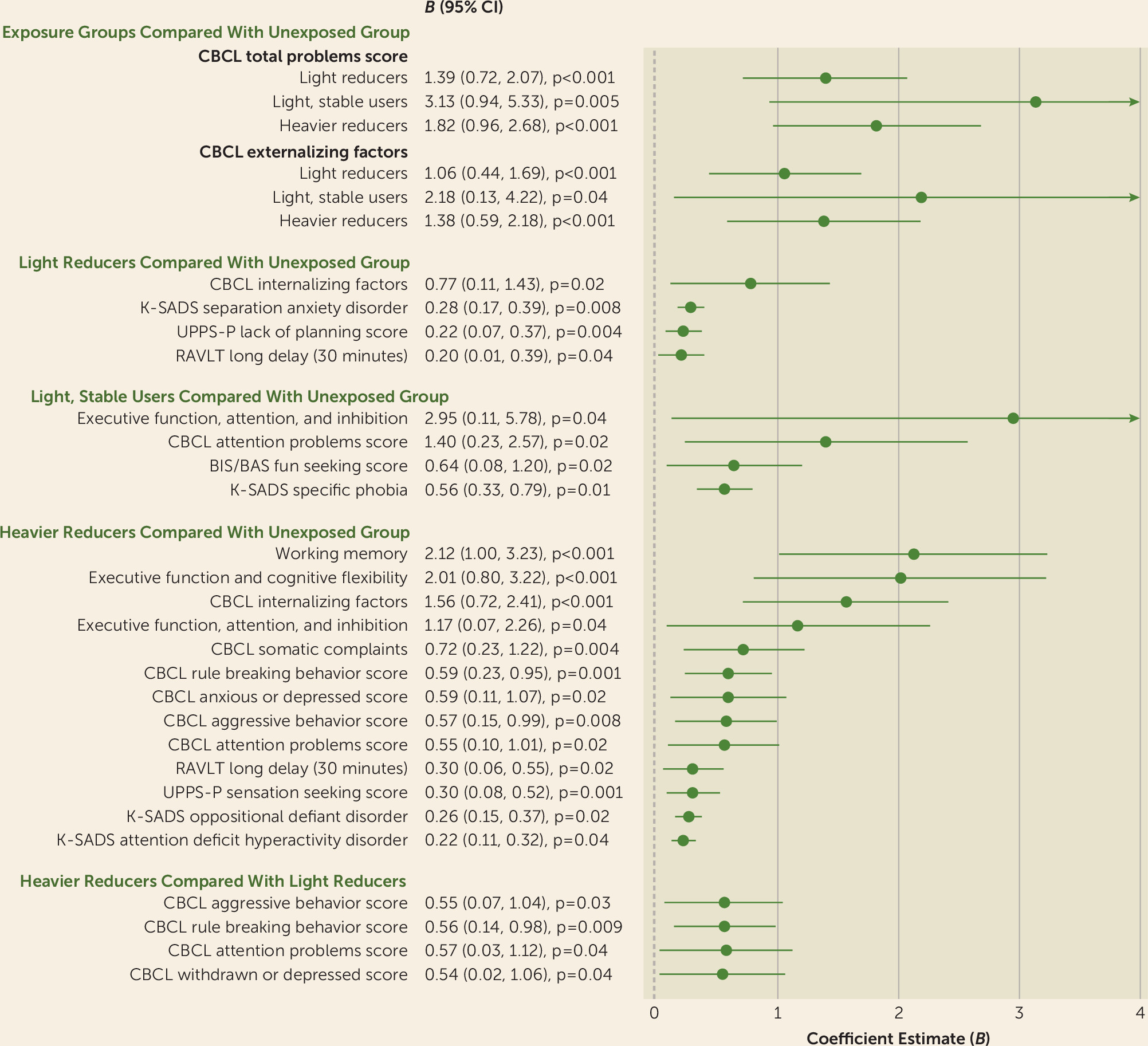
Prenatal alcohol exposure of any severity was associated with greater psychopathology, impulsivity, and likelihood of being diagnosed with separation anxiety and oppositional defiant disorder, with some observed dose-related associations. Heavier exposure was also associated with greater withdrawn or depressed behavior, attention deficits, rule breaking, aggression, and a greater likelihood of being diagnosed with ADHD. Early, light exposure, compared with no exposure, was associated with better attention and inhibitory skills. Exposed youths also exhibited greater cerebral volume, in a dose-dependent manner, and greater volume and surface area, but not cortical thickness, throughout regions of the parietal, temporal, and occipital lobes, after accounting for potentially confounding factors. Resting-state functional connectivity was largely unaltered in these youths. Aberrant brain structure partially mediated associations between prenatal alcohol exposure and psychological, behavioral, and cognitive outcomes at baseline and at the 1-year follow-up. These reported associations passed a stringent demographic-matching protocol. Unmodifiable factors greatly contributed to the large effect sizes in the adjusted models. Of the modifiable factors, prenatal alcohol exposure was a critical determinant of brain structure, and some neurobehavioral outcomes, accounting for >50% of the explained variance by modifiable factors. The findings were in a largely substance-naive cohort of youths (99.999%), allowing for investigation of the effects of prenatal alcohol exposure on the developing brain and behavior in the absence of youths’ own substance use, which is known to affect neurodevelopment (
30).
Comparison With Other Studies
Our findings replicate previous clinical studies indicating that children exposed to alcohol in utero have higher rates of mental disorders and present with behavioral anomalies, including impulsiveness and attention deficits (
14). Results from our dose-dependent and exposure pattern analyses support the notion that the severity of psychopathology and behavioral problems depends on alcohol dose and timing of exposure. The present results are also consistent with previous reports using the ABCD cohort of associations between psychopathology, brain structure, and resting-state functional connectivity (
31,
32). Consistent with previous meta-analyses, a small, beneficial association between prenatal alcohol exposure and cognitive ability was observed (
33,
34). However, when participants were demographically matched, the vast majority of associations were no longer significant. This association may be the result of residual confounding from socioeconomic status and other demographic variables, as previously hypothesized (
33,
34). Other confounding variables not captured in this analysis may be contributing to the positive association between early, light exposure and attention and inhibition.
The long-term neurostructural and functional effects of light maternal drinking, where offspring who do not necessarily present with fetal alcohol spectrum disorder, have not been well studied. Consistent with our findings, one study has reported larger regional volume among youths prenatally exposed to alcohol relative to unexposed youths (
35). However, in contrast to our results, a common finding, when investigated both categorically and continuously, has been less volume and surface area among youths with fetal alcohol spectrum disorder and those with heavier prenatal alcohol exposure compared with unexposed youths (
7,
36). Furthermore, a previous study of youths with fetal alcohol spectrum disorder reported hypoconnectivity between numerous large-scale neurocognitive networks (
13), yet in the present study, no significant alterations in resting-state functional connectivity were observed within or between these networks (see Table S5 the
online supplement).
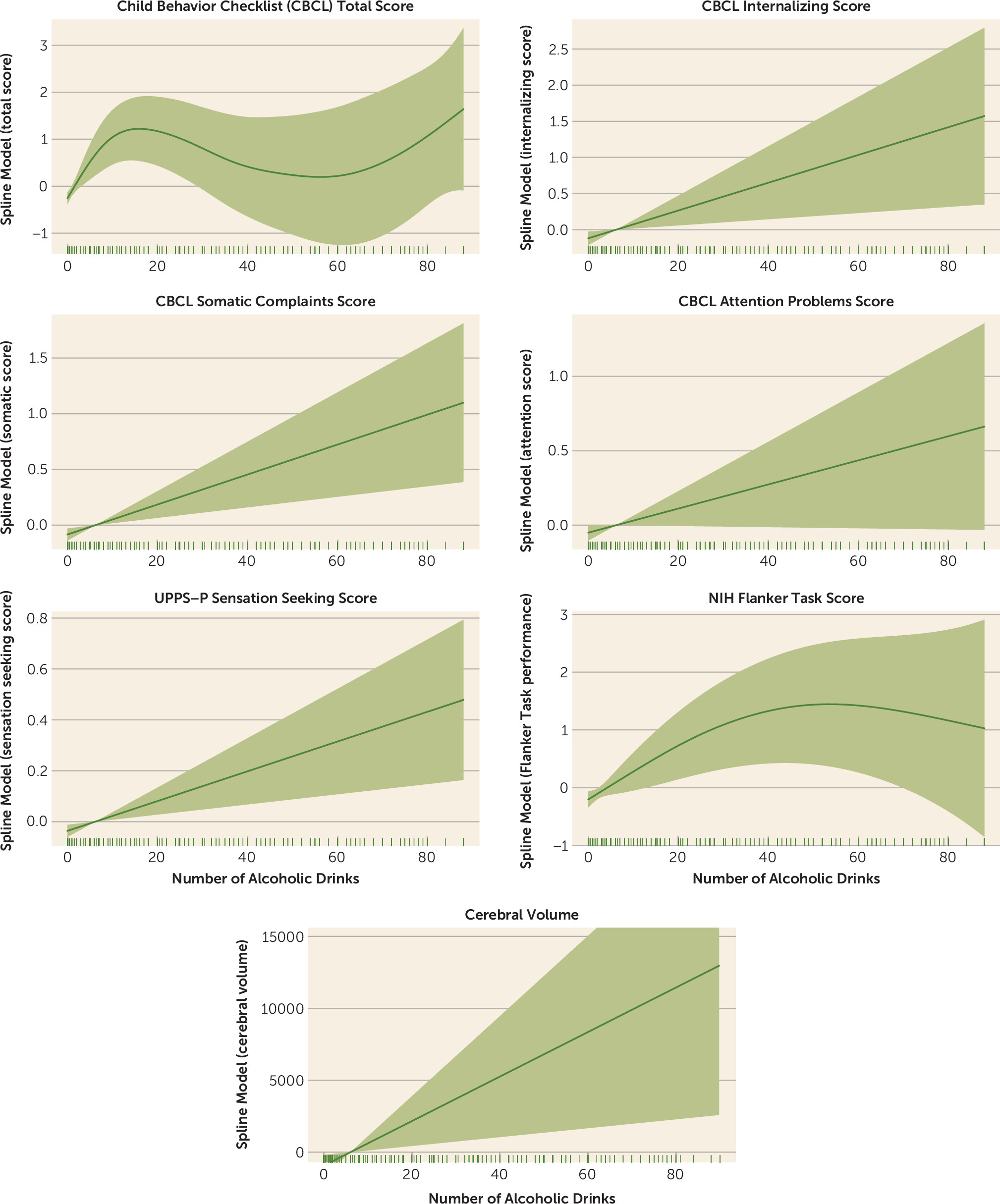
The disparate findings may be explained by the large discrepancies in clinical severity of prenatal alcohol exposure between the ABCD sample and previous cohorts. The impact of heavier prenatal alcohol exposure may have a differential effect on preadolescent brain structure and function. Interestingly, some regions of the occipital, temporal, and parietal lobes exhibited an inverted-U association between alcohol dose and volume or surface area (see Figure S1 in the online supplement). It is possible, therefore, that we would have observed reduced volume and surface area among youths exposed to heavier doses (i.e., >90 drinks consumed during pregnancy). Furthermore, potentially confounding factors in previous studies of children with heavier prenatal alcohol exposure or fetal alcohol spectrum disorder may contribute to the discrepant findings, such as greater co-occurring substance exposure, early-life stress, and quality of parental care. Importantly, our findings suggest that youths exposed to even light alcohol doses in utero exhibit widespread differences in brain structure, when compared with unexposed youths.
Finally, our results are consistent with previous studies of children with fetal alcohol spectrum disorder that have linked behavioral, psychological, and cognitive outcomes to changes in brain structure (
10). However, our study is the first to test and identify inconsistent mediation between these variables (
29). Similar to previous conclusions drawn on the effects of prenatal alcohol exposure (
37), our results suggest that there is no safe threshold for alcohol consumption during pregnancy.
Interpretation and Potential Biological Mechanisms Underlying Neurobehavioral Outcomes
Alcohol is a known teratogen in utero, and it is thought to affect regions of the developing fetal brain via neural proliferation and migration errors, hypoxia, and cell death (
38). The teratogenic effects likely differ as a result of dose, frequency, and timing of exposure and may vary across brain regions. Our findings demonstrate that there are complex effects of prenatal alcohol exposure on offspring development. Here, we provide four potential interpretations of mechanisms underlying associations between prenatal alcohol exposure, differences in brain structure, and neurobehavioral consequences.
First, our results may reflect a compensatory response of some brain regions attempting to counter the effects of other, poorer functioning regions affected by low alcohol doses (
39). Our inconsistent mediation findings provide some support for this interpretation, where greater brain volume and surface area were associated with better neurobehavioral outcomes, yet youths who were exposed to alcohol in utero exhibited greater volume and surface area but more neurobehavioral problems at baseline and follow-up. Despite a potential compensatory response of the brain to counter the effects of relatively low doses of alcohol, these youths continue to show subtle, yet poorer, psychological and behavioral outcomes through early life.
Second, our findings may also suggest that relatively light prenatal alcohol exposure may result in slightly atypical neurodevelopment. Such exposure may slow or alter the overall process of gray matter maturation, where greater absolute volume and surface area in exposed youths represent delayed or incomplete cortical pruning compared with this process in unexposed, prepubertal youths (
40). Consistent with this hypothesis, we observed this trend largely in regions where gray matter loss in unexposed children progresses linearly from childhood through adolescence (
41). Typically among this age group, the left hemisphere matures earlier than the right (i.e., left hemisphere gray matter loss prior to right hemisphere gray matter loss;
41,
42). Greater volume and surface area among exposed youths in left posterior cortices known to develop most rapidly between childhood and adolescence provide further support of delayed development. Examining the developmental trajectories of this cohort when multiple waves of imaging data are available will provide further insight into whether atypical development is occurring among exposed youths.
Third, the inconsistent mediation findings may also be partly capturing the effects of the inverted-U associations between total alcohol dose and regional brain volume and surface area. Youths exposed to greater alcohol doses (i.e., approximately 90 drinks consumed during pregnancy) exhibited greater psychopathology and behavioral problems between ages 9 and 10 than youths exposed to lighter doses (i.e., approximately 40 drinks), and these more heavily exposed youths exhibited lower volume and surface area in regions of the parietal and temporal lobes than youths exposed to lighter doses.
Lastly, there may be other critical changes resulting from prenatal alcohol exposure that mediate associations with brain structure differences and psychological and behavioral outcomes. For example, ethanol provokes a wide range of epigenetic modifications, including altered DNA and histone methylation, which persist from birth through childhood (
43). Animal studies suggest that prenatal alcohol exposure affects DNA methylation through antagonistic effects on methyl donors, such as folate, and via long-lasting changes in gene expression (
43). Preliminary evidence from studies of children with fetal alcohol spectrum disorder show genome-wide differences in DNA methylation (
44). Further research is required to examine epigenetic markers and their role in adverse outcomes among exposed youths; DNA methylation or other epigenetic markers could potentially provide objective indicators of prenatal alcohol exposure.
Limitations of our study include potential maternal underreporting of alcohol use during pregnancy, imprecise retrospective data on the timing, amount, and frequency of alcohol exposure, and absence of data on trimester-specific alcohol exposure. The effects of underreporting by mothers who indicated alcohol use during pregnancy may have inflated the observed associations, while underreporting by mothers who indicated no alcohol use when they did in fact consume alcohol would have attenuated the associations toward the null. Future studies may benefit from interviewing an independent reporter of prenatal maternal alcohol use. Furthermore, data were not available on mothers who regularly consumed less than a full unit of alcohol. Therefore, youths exposed to this pattern of drinking would have been included in the unexposed group, potentially diluting outcome effects. Despite the large sample size, there were relatively few cases of youths exposed to stable light drinking throughout pregnancy, and too few cases of stable heavier drinking or increased consumption throughout pregnancy, to examine the impact on offspring. There is a larger body of existing evidence based on the consequences of heavier alcohol exposure (
7). The small sample size of youths exposed to light, stable drinking throughout pregnancy resulted in wider variance in outcome measures and may underestimate the true impact. Other notable explanatory variables of early life that may influence the observed associations between prenatal alcohol exposure and neurobehavioral outcomes include childhood adversity and quality of parental care. These variables may contribute to mediating effects of neurodevelopment and possible epigenetic modifications (
45). The baseline ABCD Study protocol did not capture these variables, although future waves will. Longitudinal analyses of this cohort should consider these variables as possible confounding factors. In addition, we did not examine the effect of preconception paternal alcohol exposure on preadolescent brain structure, and this should be explored in future studies.
In conclusion, relatively light levels of prenatal alcohol exposure were associated with small yet significantly greater psychological and behavioral problems, including internalizing and externalizing psychopathology, attention deficits, and impulsiveness. These outcomes were linked to differences in cerebral and regional brain volume and regional surface area among exposed youths ages 9 to 10 years. Examination of dose-dependent relationships and light alcohol exposure patterns during pregnancy shows that children with even the lowest levels of exposure demonstrate poorer psychological and behavioral outcomes as they enter adolescence. Associations preceded offspring alcohol use and were robust to the inclusion of potential confounding factors and during stringent demographic matching procedures, increasing the plausibility of the findings. Women should continue to be advised to abstain from alcohol consumption from conception throughout pregnancy.